Behavioural Brain Research ( IF 2.7 ) Pub Date : 2021-09-15 , DOI: 10.1016/j.bbr.2021.113582 Lorrane Kelle da Silva Moreira 1 , Rafaela Ribeiro Silva 2 , Dayane Moreira da Silva 1 , Mirella Andrade Silva Mendes 3 , Adriane Ferreira de Brito 1 , Flávio Souza de Carvalho 3 , Germán Sanz 4 , Marcella Ferreira Rodrigues 4 , Artur Christian Garcia da Silva 5 , Douglas Vieira Thomaz 3 , Valéria de Oliveira 3 , Boniek Gontijo Vaz 4 , Luciano Morais Lião 6 , Marize Campos Valadares 5 , Eric de Souza Gil 3 , Elson Alves Costa 1 , François Noël 2 , Ricardo Menegatti 3
|
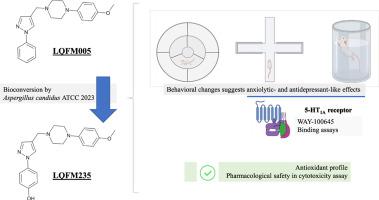
The current treatments available for anxiety and depression are only palliative. Full remission has remained elusive, characterizing unmet medical needs. In the scope of an academic drug discovery program, we describe here the design, synthesis, in vitro metabolism prediction and pharmacological characterization of a new piperazine compound, 1-(4-methoxyphenyl)−4-((1-phenyl-1H-pyrazol-4-yl)methyl)piperazine (LQFM005), and of its main putative metabolite, 4-(4-((4-(4-methoxyphenyl)piperazin-1-yl)methyl)− 1H-pyrazol-1-yl)phenol (LQFM235). The production of the metabolite was initially performed by in vitro biotransformation of LQFM005 using Aspergillus candidus and then by chemical synthesis. Oral administration of either 12 or 24 µmol/kg LQFM005 to mice did not affect spontaneous locomotor activity but increased the time spent in the center of the open field. Both LQFM005 and LQFM235 (24 µmol/kg) increased the time spent by the mice in the open arms of the elevated plus maze (EPM), a good indication of anxiolytic-like effect, and decreased the immobility time in the forced swimming test (FST), suggesting an antidepressant-like effect. The previous administration of WAY-100635 (a 5-HT1A antagonist) abolished the effects of LQFM005 in both EPM and FST. Binding experiments showed that LQFM005 and its metabolite bind to the 5-HT1A receptor with a moderate affinity (Ki around 5–9 µM). The two compounds are relatively safe, as indicated by cytotoxic assessment using the 3T3 fibroblast cell line and estimated LD50 around 600 mg/kg. In conclusion, oral administration of the newly synthesized phenylpiperazines produced anxiolytic- and antidepressant-like effects in behavioral tests, putatively in part through the activation of 5-HT1A receptors.
中文翻译:

新型苯基哌嗪衍生物 LQFM005 及其羟基化代谢物对小鼠的抗焦虑和抗抑郁作用
目前可用于焦虑和抑郁的治疗方法只是姑息性的。完全缓解仍然难以捉摸,这是未满足的医疗需求的特征。在学术药物发现计划的范围内,我们在此描述了一种新型哌嗪化合物 1-(4-methoxyphenyl)−4-((1-phenyl-1 H - pyrazol-4-yl)methyl)piperazine (LQFM005) 及其主要推定代谢物 4-(4-((4-(4-methoxyphenyl)piperazin-1-yl)methyl)− 1 H -pyrazol-1-基)苯酚(LQFM235)。代谢物的生产最初是通过使用念珠菌对 LQFM005 进行体外生物转化来进行的然后通过化学合成。给小鼠口服 12 或 24 µmol/kg LQFM005 不会影响自发运动活动,但会增加在开阔场地中心停留的时间。LQFM005 和 LQFM235 (24 µmol/kg) 都增加了小鼠在高架十字迷宫 (EPM) 的张开臂中花费的时间,这是抗焦虑样作用的良好指示,并减少了强迫游泳试验中的不动时间。 FST),表明具有抗抑郁作用。之前施用的 WAY-100635(一种 5-HT 1A拮抗剂)消除了 LQFM005 在 EPM 和 FST 中的作用。结合实验表明 LQFM005 及其代谢物与 5-HT 1A受体具有中等亲和力(K i大约 5–9 µM)。这两种化合物相对安全,如使用 3T3 成纤维细胞系的细胞毒性评估所示,估计 LD 50约为 600 mg/kg。总之,口服新合成的苯哌嗪在行为测试中产生抗焦虑和抗抑郁样作用,推测部分是通过激活 5-HT 1A受体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号