当前位置:
X-MOL 学术
›
J. Mater. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
BN-Substituted coronene diimide donor–acceptor–donor triads: photophysical, (spectro)-electrochemical studies and Lewis behavior
Journal of Materials Chemistry C ( IF 5.7 ) Pub Date : 2021-09-15 , DOI: 10.1039/d1tc03034e Jonas Hoffmann 1, 2, 3 , Denis Jacquemin 4 , Muriel Hissler 3 , Anne Staubitz 1, 2
Journal of Materials Chemistry C ( IF 5.7 ) Pub Date : 2021-09-15 , DOI: 10.1039/d1tc03034e Jonas Hoffmann 1, 2, 3 , Denis Jacquemin 4 , Muriel Hissler 3 , Anne Staubitz 1, 2
Affiliation
|
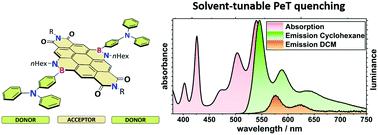
Boron/nitrogen substituted polyaromatic hydrocarbons (PAHs) are unique materials, with similar molecular structures to their carbon/carbon analogs, but different electronic properties. We report how these may be tuned by substitution at the B and/or N atoms: the BN-PAH analogues we investigated are BN-substituted coronene diimide (BNCDI) acceptors, combined with electron-rich (hetero)arene substituents (donors) on the boron atoms at either side of the BNCDI, resulting in donor–acceptor–donor (D–A–D) triads. In comparison to the all-carbon coronene diimide, the implementation of two BN units led to a bathochromic shift of absorption/emission (44 nm) and luminescence quantum yields close to unity. The uniqueness of the BN-based D–A–D motif became clear as the substitution effect of the (hetero)arene substituents was investigated. The strong electron-donating triphenylamine motif had a minor influence on the absorption behavior but showed strongly solvent-dependent luminescence properties. This could be attributed to an intramolecular photoinduced electron transfer (PeT) process which was supported by a Rehm–Weller analysis and density functional theory computations. This process was not observed for the other substituents. Moreover, we probed the influence of the aryl substituent on the B–N bond itself by using (spectro)electrochemistry and analyzed the Lewis behavior of the BN unit. The species that formed showed strong absorptions across the whole UV/vis/NIR region.
中文翻译:

BN-取代的晕苯二亚胺供体-受体-供体三元组:光物理、(光谱)-电化学研究和路易斯行为
硼/氮取代的多环芳烃 ( PAH ) 是独特的材料,其分子结构与其碳/碳类似物相似,但电子特性不同。我们报告了如何通过 B 和/或 N 原子上的取代来调整这些:我们研究的 BN-PAH 类似物是 BN 取代的晕苯二亚胺( BNCDI ) 受体,结合富电子(杂)芳烃取代基(供体)BNCDI两侧的硼原子,导致供体 - 受体 - 供体(D - A - D) 黑社会。与全碳晕苯二亚胺相比,两个 BN 单元的实施导致吸收/发射的红移 (44 nm) 和发光量子产率接近统一。随着对(杂)芳烃取代基的取代效应的研究,基于 BN 的D - A - D基序的独特性变得清晰起来。强供电子三苯胺基序对吸收行为的影响很小,但显示出强烈的溶剂依赖性发光特性。这可能归因于分子内光致电子转移(PeT) 过程得到了 Rehm-Weller 分析和密度泛函理论计算的支持。对于其他取代基没有观察到这个过程。此外,我们通过使用(光谱)电化学探讨了芳基取代基对 B-N 键本身的影响,并分析了 BN 单元的路易斯行为。形成的物种在整个 UV/vis/NIR 区域显示出强烈的吸收。
更新日期:2021-09-15
中文翻译:

BN-取代的晕苯二亚胺供体-受体-供体三元组:光物理、(光谱)-电化学研究和路易斯行为
硼/氮取代的多环芳烃 ( PAH ) 是独特的材料,其分子结构与其碳/碳类似物相似,但电子特性不同。我们报告了如何通过 B 和/或 N 原子上的取代来调整这些:我们研究的 BN-PAH 类似物是 BN 取代的晕苯二亚胺( BNCDI ) 受体,结合富电子(杂)芳烃取代基(供体)BNCDI两侧的硼原子,导致供体 - 受体 - 供体(D - A - D) 黑社会。与全碳晕苯二亚胺相比,两个 BN 单元的实施导致吸收/发射的红移 (44 nm) 和发光量子产率接近统一。随着对(杂)芳烃取代基的取代效应的研究,基于 BN 的D - A - D基序的独特性变得清晰起来。强供电子三苯胺基序对吸收行为的影响很小,但显示出强烈的溶剂依赖性发光特性。这可能归因于分子内光致电子转移(PeT) 过程得到了 Rehm-Weller 分析和密度泛函理论计算的支持。对于其他取代基没有观察到这个过程。此外,我们通过使用(光谱)电化学探讨了芳基取代基对 B-N 键本身的影响,并分析了 BN 单元的路易斯行为。形成的物种在整个 UV/vis/NIR 区域显示出强烈的吸收。











































 京公网安备 11010802027423号
京公网安备 11010802027423号