Computational Materials Science ( IF 3.1 ) Pub Date : 2021-09-14 , DOI: 10.1016/j.commatsci.2021.110860 Sunday Temitope Oyinbo 1 , Tien-Chien Jen 1
|
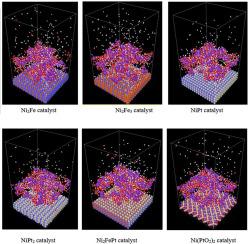
In recent years, significant attention has been paid to the possible use of hydrogen (H2) as a renewable energy source. The formation of H2, in particular, is appealing by Ni-based-catalyzed activity in an alkaline potassium hydroxide (KOH) solution at room temperatures. However, a broader understanding of the reactions at the catalyst surface needs to be sought at the atomic level. In this research, a comparative and systematic study of nickel-based heterometallic catalysts in an alkaline potassium hydroxide (KOH) solution for H2 generation with the aid of ReaxFF potential is performed using reactive molecular dynamics (RMD) simulations. The interface composition of nickel catalyst was systematically modulated to include transition metals such as iron, platinum, and their oxides. All nickel-based transition metals and their oxides are equally active while influencing the catalytic reaction. Ni-Fe and Ni-Pt impose major promoting effects on the Ni-based catalyst, with an improvement in the generation rate of output of H2 when compared to the Ni-Fe-Pt heterometallic catalyst. On the other hand, only a marginal improvement in the catalytic effectiveness of the Ni-based catalyst is evident with Ni-Fe-O and Ni-Pt-O catalyst. The molecular proportion of the metal/(Ni + oxide) was varied within the catalyst to investigate the impact of metal combination and concentration on the alloy catalytic performance. This structural variation also explained the role of each second metal and their oxide involved in an alkaline KOH electron exchange process. The second metal promoting effects are mainly summarized in terms of the ability to serve as an electrophilic site for enhanced group absorption, a large area of active surface, amorphous characteristic of the alloy catalyst, and interaction of the electrons with the Ni active metal.
中文翻译:

镍基异金属催化剂在碱性 KOH 溶液中析氢的反应分子动力学模拟
近年来,人们对氢(H 2)作为可再生能源的可能用途给予了极大的关注。H 2的形成,尤其是在室温下碱性氢氧化钾 (KOH) 溶液中的 Ni 基催化活性很吸引人。然而,需要在原子水平上寻求对催化剂表面反应的更广泛理解。在本研究中,对碱性氢氧化钾 (KOH) 溶液中的 H 2镍基异金属催化剂进行了比较和系统的研究。借助反应性分子动力学 (RMD) 模拟,借助 ReaxFF 电位生成。镍催化剂的界面组成被系统地调节以包括过渡金属,例如铁、铂及其氧化物。所有镍基过渡金属及其氧化物在影响催化反应的同时具有同等活性。Ni-Fe和Ni-Pt对Ni基催化剂有很大的促进作用,提高了H 2的产出率与 Ni-Fe-Pt 异金属催化剂相比。另一方面,Ni-Fe-O 和 Ni-Pt-O 催化剂对 Ni 基催化剂的催化效率仅略有改善。金属/(Ni + 氧化物)的分子比例在催化剂内变化,以研究金属组合和浓度对合金催化性能的影响。这种结构变化也解释了每种第二种金属及其氧化物在碱性 KOH 电子交换过程中的作用。第二种金属促进作用主要概括为作为亲电子位点的能力。基团吸收、大面积的活性表面、合金催化剂的非晶特性以及电子与 Ni 活性金属的相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号