Computational Materials Science ( IF 3.1 ) Pub Date : 2021-09-14 , DOI: 10.1016/j.commatsci.2021.110863 Di Zhao 1 , Feng Liu 1 , Xiangmei Duan 2 , Deyan Sun 1, 3
|
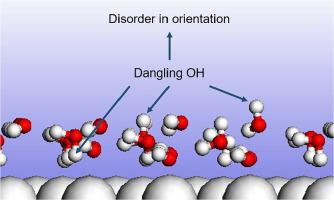
Using the first-principles calculation, we have systematically investigated the orientation of dangling OH-bonds in the first adsorbed water layer on close packed surface of noble metals (gold, platinum and palladium). We find that, the distribution of up and down dangling OH-bonds can strongly change the adsorption stability. A specific distribution of dangling OH-bonds is always indispensable in various adsorption patterns for its stability. The in-plane arrangement of water molecules also has important influence on the orientation of dangling OH-bonds. More importantly, the disorder in the orientation (up or down) of dangling OH manifests a kind of zero-temperature residual entropy in some absorption structures. In essence, the residual entropy arises from the competition between water-water and water-metal interactions, which is different from the counterpart in ice crystals.
中文翻译:

贵金属表面第一层水层中悬空 OH 键的内在无序
使用第一性原理计算,我们系统地研究了贵金属(金、铂和钯)密堆积表面上第一吸附水层中悬空 OH 键的取向。我们发现,上下悬空 OH 键的分布可以强烈改变吸附稳定性。悬空 OH 键的特定分布因其稳定性在各种吸附模式中总是必不可少的。水分子的平面排列也对悬空 OH 键的取向有重要影响。更重要的是,悬空 OH 方向(向上或向下)的无序在某些吸收结构中表现出一种零温度残余熵。本质上,残余熵来自水-水和水-金属相互作用之间的竞争,







































 京公网安备 11010802027423号
京公网安备 11010802027423号