Chemosphere ( IF 8.1 ) Pub Date : 2021-09-15 , DOI: 10.1016/j.chemosphere.2021.132229 Dawany Dionisio 1 , Manuel A Rodrigo 2 , Artur J Motheo 3
|
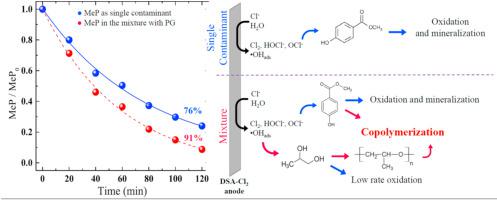
Endocrine disrupting compounds (EDCs) are one of the many classes of harmful pollutants frequently found in water resources. Even at low concentrations, EDCs might accumulate in the organisms and interfere on numerous processes controlled by hormones. Parabens, for example, are preservatives widely used in pharmaceutical and cosmetic industries, but several studies related them to human breast cancer. It is well-known that electrochemical technologies are an efficient alternative for wastewater treatment, promoting the appropriate destruction of EDCs. However, most studies are applied to single target contaminant solutions, which may neglect the impact from co-exited inorganic/organic pollutants. Based on that, this study aimed to elucidate the interfering effects of two target organic contaminants of very different nature during electrochemical mediated process. For that, methyl paraben (MeP) and propylene glycol (PG) were selected as models of aromatic/phenolic and carboxylate compounds versus low-molecular aliphatic alcohols. These two compounds are often together used in preservative blends and cosmetic/pharmaceutical formulations. PG is not a harmful chemical, but it is present in several types of effluents in relatively high concentrations. Thus, it may interfere on the degradation of numerous pollutants of low concentrations. The electrochemical treatment of a mixture containing 100 mg L−1 MeP +1000 mg L−1 PG showed that both contaminants suffered interfering effects. The presence of MeP negatively interfered on PG degradation; the carboxylate compound is more easily oxidized even at lower molecular concentration. On the other hand, the presence of PG showed an unexpected positive effect on MeP degradation, that was not reflected on its mineralization. The results indicate that in addition to the expected effect of anodic competition, polymerization and copolymerization reactions may also occur in the studied system. The use of an acidic buffer medium increased the removal of both contaminants and favored the oxidation pathway over the polymerization. In this case, the increase in the removal was reflected in the mineralization process, which increased up to 6 times when the mixture was treated in the buffered medium.
中文翻译:

对羟基苯甲酸甲酯和丙二醇混合物的电化学降解:竞争氧化和 pH 稳定性的干扰作用
内分泌干扰物 (EDC) 是水资源中常见的多种有害污染物之一。即使在低浓度下,EDCs 也可能在生物体内积累并干扰由激素控制的许多过程。例如,对羟基苯甲酸酯是一种广泛用于制药和化妆品行业的防腐剂,但有几项研究将它们与人类乳腺癌联系起来。众所周知,电化学技术是废水处理的有效替代方法,可促进 EDC 的适当破坏。然而,大多数研究都应用于单一目标污染物溶液,这可能会忽略共存无机/有机污染物的影响。基于此,本研究旨在阐明两种性质截然不同的目标有机污染物在电化学介导过程中的干扰作用。为此,选择对羟基苯甲酸甲酯 (MeP) 和丙二醇 (PG) 作为芳香族/酚类和羧酸酯化合物的模型与低分子脂肪醇相比。这两种化合物通常一起用于防腐剂混合物和化妆品/药物配方中。PG 不是一种有害化学物质,但它以相对较高的浓度存在于几种类型的污水中。因此,它可能会干扰许多低浓度污染物的降解。含有 100 mg L -1 MeP +1000 mg L -1的混合物的电化学处理PG 表明两种污染物都受到干扰。MeP 的存在对 PG 降解有负面影响;即使在较低的分子浓度下,羧酸盐化合物也更容易被氧化。另一方面,PG 的存在对 MeP 降解显示出意想不到的积极影响,这并未反映在其矿化上。结果表明,除了预期的阳极竞争效应外,所研究的体系中还可能发生聚合和共聚反应。酸性缓冲介质的使用增加了两种污染物的去除,并有利于氧化途径而不是聚合反应。在这种情况下,去除量的增加反映在矿化过程中,当混合物在缓冲介质中处理时,去除量增加了 6 倍。











































 京公网安备 11010802027423号
京公网安备 11010802027423号