Cell Reports Physical Science ( IF 8.9 ) Pub Date : 2021-09-15 , DOI: 10.1016/j.xcrp.2021.100579 Yiming Tang 1 , Santu Bera 2 , Yifei Yao 1 , Jiyuan Zeng 1 , Zenghui Lao 1 , Xuewei Dong 1 , Ehud Gazit 2 , Guanghong Wei 1
|
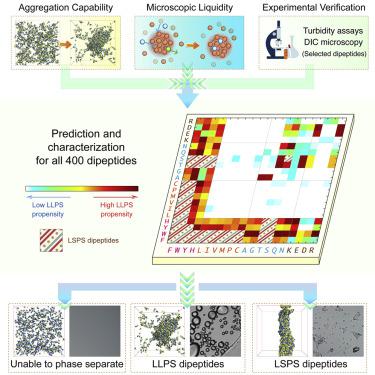
Liquid-liquid phase separation (LLPS) of proteins mediates the assembly of biomolecular condensates involved in physiological and pathological processes. Identifying the minimalistic building blocks and the sequence determinant of protein phase separation is an urgent need but remains challenging partially due to lack of methodologies to characterize the phase behavior. Here, we demonstrate computational tools to efficiently quantify the microscopic fluidity of liquid condensates and the temperature-dependent phase diagram of peptides. We comprehensively explore the LLPS abilities of all 400 dipeptide combinations of coded amino acids and observe the occurrences of spontaneous LLPS in three categories of dipeptides. Our predictions are validated by turbidity assays and differential interference contrast (DIC) microscopy. We demonstrate that dipeptides, minimal but complete, possess multivalent interactions sufficient for LLPS, suggesting LLPS is a general property of peptides and proteins, independent of their sequence length. This study paves the way for the prediction and characterization of peptide phase behavior at the molecular level.
中文翻译:

极简肽液液相分离的预测与表征
蛋白质的液-液相分离 (LLPS) 介导了参与生理和病理过程的生物分子凝聚物的组装。确定蛋白质相分离的简约构建块和序列决定因素是一项紧迫的需求,但部分由于缺乏表征相行为的方法,仍然具有挑战性。在这里,我们展示了计算工具,以有效量化液体冷凝物的微观流动性和肽的温度相关相图。我们全面探索了所有 400 种编码氨基酸二肽组合的 LLPS 能力,并观察了三类二肽中自发性 LLPS 的发生。我们的预测得到了浊度测定和微分干涉对比 (DIC) 显微镜的验证。我们证明二肽,最小但完整,具有足以用于 LLPS 的多价相互作用,表明 LLPS 是肽和蛋白质的一般特性,与它们的序列长度无关。该研究为在分子水平上预测和表征肽相行为铺平了道路。



























 京公网安备 11010802027423号
京公网安备 11010802027423号