Cell ( IF 45.5 ) Pub Date : 2021-09-15 , DOI: 10.1016/j.cell.2021.09.015 Alexandra C Walls 1 , Marcos C Miranda 2 , Alexandra Schäfer 3 , Minh N Pham 2 , Allison Greaney 4 , Prabhu S Arunachalam 5 , Mary-Jane Navarro 1 , M Alejandra Tortorici 6 , Kenneth Rogers 7 , Megan A O'Connor 8 , Lisa Shirreff 7 , Douglas E Ferrell 7 , John Bowen 1 , Natalie Brunette 2 , Elizabeth Kepl 2 , Samantha K Zepeda 1 , Tyler Starr 9 , Ching-Lin Hsieh 10 , Brooke Fiala 2 , Samuel Wrenn 2 , Deleah Pettie 2 , Claire Sydeman 2 , Kaitlin R Sprouse 1 , Max Johnson 2 , Alyssa Blackstone 2 , Rashmi Ravichandran 2 , Cassandra Ogohara 2 , Lauren Carter 2 , Sasha W Tilles 11 , Rino Rappuoli 12 , Sarah R Leist 3 , David R Martinez 3 , Matthew Clark 13 , Roland Tisch 14 , Derek T O'Hagan 15 , Robbert Van Der Most 16 , Wesley C Van Voorhis 11 , Davide Corti 17 , Jason S McLellan 10 , Harry Kleanthous 18 , Timothy P Sheahan 3 , Kelly D Smith 19 , Deborah H Fuller 8 , Francois Villinger 7 , Jesse Bloom 4 , Bali Pulendran 5 , Ralph S Baric 3 , Neil P King 2 , David Veesler 1
|
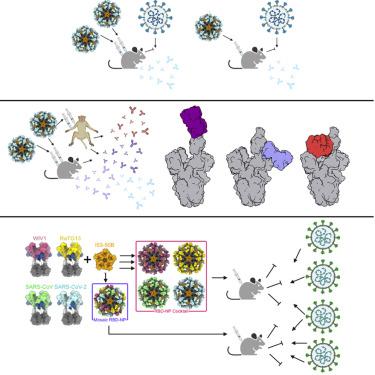
Understanding vaccine-elicited protection against SARS-CoV-2 variants and other sarbecoviruses is key for guiding public health policies. We show that a clinical stage multivalent SARS-CoV-2 spike receptor-binding domain nanoparticle (RBD-NP) vaccine protects mice from SARS-CoV-2 challenge after a single immunization, indicating a potential dose-sparing strategy. We benchmarked serum neutralizing activity elicited by RBD-NPs in non-human primates against a lead prefusion-stabilized SARS-CoV-2 spike (HexaPro) using a panel of circulating mutants. Polyclonal antibodies elicited by both vaccines are similarly resilient to many RBD residue substitutions tested, although mutations at and surrounding position 484 have negative consequences for neutralization. Mosaic and cocktail nanoparticle immunogens displaying multiple sarbecovirus RBDs elicit broad neutralizing activity in mice and protect mice against SARS-CoV challenge even in the absence of SARS-CoV RBD in the vaccine. This study provides proof of principle that multivalent sarbecovirus RBD-NPs induce heterotypic protection and motivates advancing such broadly protective sarbecovirus vaccines to the clinic.
中文翻译:

通过受体结合域纳米颗粒疫苗引发广泛的保护性沙病毒免疫
了解疫苗对 SARS-CoV-2 变种和其他 sarbeco 病毒的保护作用是指导公共卫生政策的关键。我们证明,临床阶段多价 SARS-CoV-2 刺突受体结合域纳米颗粒 (RBD-NP) 疫苗在单次免疫后可保护小鼠免受 SARS-CoV-2 攻击,这表明一种潜在的剂量节约策略。我们使用一组循环突变体对 RBD-NP 在非人类灵长类动物中引起的血清中和活性与先导预融合稳定的 SARS-CoV-2 刺突 (HexaPro) 进行了基准测试。尽管 484 位及其周围的突变对中和有负面影响,但两种疫苗引发的多克隆抗体对许多测试的 RBD 残基取代具有相似的弹性。显示多种 sarbecovirus RBD 的马赛克和鸡尾酒纳米颗粒免疫原在小鼠中引发广泛的中和活性,即使在疫苗中不存在 SARS-CoV RBD 的情况下,也能保护小鼠免受 SARS-CoV 的攻击。这项研究提供了多价 sarbecovirus RBD-NP 诱导异型保护的原理证明,并推动将这种具有广泛保护性的 sarbecovirus 疫苗推向临床。











































 京公网安备 11010802027423号
京公网安备 11010802027423号