Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2021-09-15 , DOI: 10.1016/j.jcis.2021.09.060 Xiaolan Li 1 , Yang Liu 1 , Jinliang Zhu 1 , Panagiotis Tsiakaras 2 , Pei Kang Shen 1
|
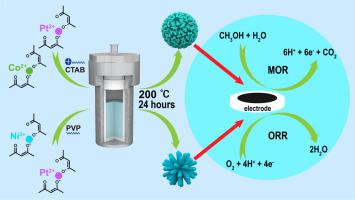
Herein, we introduce a facile approach to synthesize a unique class of Pt-M (M = Ni, Co) catalysts with a nanoflower structure for boosting both oxygen reduction reaction (ORR) and methanol oxidation reaction (MOR). By controlling the surface-active agents, we modified the functional groups surrounding the Pt atoms, tuned the alloying of Pt and the transition metals Ni and Co, and prepared two different kinds of nanodendrites. Their successful synthesis depends on the selection and amount of surfactants (hexadecyltrimethylammonium bromide (CTAB), Polyvinylpyrrolidone (PVP)). Besides, by controlling reaction time, we also explored the forming procedures for Pt-Co globularia nanodendrite (Pt-Co GND) and Pt-Ni petalody nanodendrite (Pt-Ni PND). Our investigation highlights the importance of complex nanoarchitecture, which enables surface and interface modification to achieve excellent catalytic performance in fuel cell electrocatalysis. The characterization of the as-prepared catalysts reveals a high electrochemical surface area and mass activity (2041 mand 950 m for Pt-Co GND and Pt-Ni PND, respectively, for ORR). Furthermore, Pt-Co GND showed a high MOR activity, with a mass activity value recorded at 1615 m which is far superior to that for Pt/C. Moreover, both catalysts retain high activity after accelerated durability tests (ADTs). The electron transfer number was calculated by performing the rotating ring-disk electrode (RRDE) measurements. Due to abundant active sites of Pt, both Pt-Co GND and Pt-Ni PND exhibit a 4e− pathway for ORR with electron transfer number of >3.95.
中文翻译:

自组装 Pt-M (M = Co, Ni) 纳米花增强氧还原和甲醇氧化反应
在此,我们引入了一种简便的方法来合成一类独特的具有纳米花结构的 Pt-M (M = Ni, Co) 催化剂,用于促进氧还原反应 (ORR) 和甲醇氧化反应 (MOR)。通过控制表面活性剂,我们修饰了 Pt 原子周围的官能团,调整了 Pt 与过渡金属 Ni 和 Co 的合金化,并制备了两种不同的纳米枝晶。它们的成功合成取决于表面活性剂(十六烷基三甲基溴化铵 (CTAB)、聚乙烯吡咯烷酮 (PVP))的选择和用量。此外,通过控制反应时间,我们还探索了 Pt-Co 球状纳米枝晶 (Pt-Co GND) 和 Pt-Ni 花瓣状纳米枝晶 (Pt-Ni PND) 的形成过程。我们的调查强调了复杂纳米结构的重要性,使表面和界面改性能够在燃料电池电催化中实现优异的催化性能。所制备催化剂的表征显示出高电化学表面积和质量活性(2041 m和 950 m分别用于 Pt-Co GND 和 Pt-Ni PND,用于 ORR)。此外,Pt-Co GND 表现出较高的 MOR 活性,质量活性值记录在 1615 m远优于 Pt/C。此外,两种催化剂在加速耐久性测试 (ADT) 后仍保持高活性。通过执行旋转环盘电极 (RRDE) 测量计算电子转移数。由于 Pt 的大量活性位点,Pt-Co GND 和 Pt-Ni PND 都表现出 4e -电子转移数大于 3.95 的 ORR 途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号