Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2021-09-15 , DOI: 10.1016/j.jinorgbio.2021.111602 Sunney I. Chan , Wei-hau Chang , Shih-Hsin Huang , Hsin-Hung Lin , Steve S.-F. Yu
|
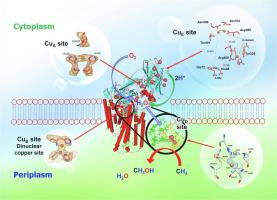
In this focused review, we portray the recently reported 2.5 Å cyro-EM structure of the particulate methane monooxygenase (pMMO) from M. capsulatus (Bath). The structure of the functional holo-pMMO near atomic resolution has uncovered the sites of the copper cofactors including the location of the active site in the enzyme. The three coppers seen in the original X-ray crystal structures of the enzyme are now augmented by additional coppers in the transmembrane domain as well as in the water-exposed C-terminal subdomain of the PmoB subunit. The cryo-EM structure offers the first glimpse of the catalytic machinery capable of methane oxidation with high selectivity and efficiency. The findings are entirely consistent with the biochemical and biophysical findings previously reported in the literature, including the chemistry of hydrocarbon hydroxylation, regeneration of the catalyst for multiple turnovers, and the mechanism of aborting non-productive cycles to ensure kinetic competence.
中文翻译:

颗粒甲烷单加氧酶 (pMMO) 中甲烷氧化的催化机制
在这篇重点综述中,我们描绘了最近报道的来自M. capsulatus (Bath)的颗粒甲烷单加氧酶 (pMMO) 的 2.5 Å cyro-EM 结构。近原子分辨率的功能性全息-pMMO 结构揭示了铜辅因子的位点,包括酶中活性位点的位置。在酶的原始 X 射线晶体结构中看到的三个铜现在被跨膜结构域和暴露于水的C中的额外铜增强-PmoB 亚基的末端亚结构域。低温 EM 结构首次展示了能够以高选择性和效率进行甲烷氧化的催化机制。这些发现与文献中先前报道的生化和生物物理发现完全一致,包括烃羟基化的化学反应、多次转换催化剂的再生以及中止非生产循环以确保动力学能力的机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号