当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alkaline Anion Exchange Membrane from Alkylated Polybenzimidazole
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2021-09-15 , DOI: 10.1021/acsaem.1c01862 Balakondareddy Sana 1 , Anupam Das 1 , Manju Sharma 1 , Tushar Jana 1
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2021-09-15 , DOI: 10.1021/acsaem.1c01862 Balakondareddy Sana 1 , Anupam Das 1 , Manju Sharma 1 , Tushar Jana 1
Affiliation
|
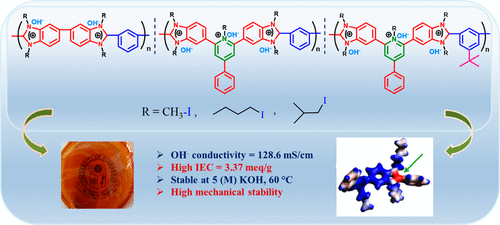
Despite the myriad literature reports on the alkaline anion exchange membrane (AAEM) in recent times, the main bottleneck yet to be resolved adequately is the development of a polymer membrane with excellent alkaline stability and high hydroxide conductivity. In order to mitigate these, in this work we have studied the influence of ion yielding alkyl group structure on the improvement of the alkaline stability and hydroxide conductivity of the AAEM with dual ion exchange sites, namely, pyridinium and imidazolium. Three different types of polymers, polybenzimidazole (PBI), pyridine bridged PBI (PyPBI), and tertiary butyl PyPBI (tBut-PyPBI), were converted to their iodide forms by alkylation of the imidazole ring by reacting with various kinds of alkyl iodides such as methyl iodide, butyl iodide, and isobutyl iodide. All the iodide forms of polybenzimidazolium membranes were transformed into the hydroxide form so as to obtain an AAEM by immersing them in KOH solution. FT-IR and 1H NMR spectroscopic studies were employed to confirm the structure of polymer, iodide forms, and KOH-loaded AAEM of all three PBI structures studied here. Ion exchange capacity (IEC), hydroxide conductivity, and thermal, mechanical, and alkaline stability of all the membranes were studied, and we found that PyPBI alkylated with butyl iodide (PyPBI-BuI) has the highest IEC, 3.37 mequiv/g, and maximum hydroxide conductivity, 128.6 mS/cm, at 80 °C among all the membranes developed in this work. All the membranes, irrespective of the polymer structure, when alkylated with isobutyl and butyl chain displayed excellent alkaline stability even in 5 M KOH aqueous solution up to 60 °C, whereas when alkylated with methyl iodide all the membranes showed poor alkaline stability even in 1 M KOH at room temperature. This observation showed the importance of the alkylated moieties structure on the alkaline stability of the AAEM. This significant stability may be due to the bulky nature of the alkyl moieties, which prevented the hydroxide ion attack on both pyridinium and imidazolium groups. Further, our computational studies using DFT calculations confirmed that the electronic factors are the major driving forces rather than the steric hindrance for such high alkaline stability in the case of longer (isobutyl, butyl) alkylated chains, particularly in the case of PyPBI and tBut-PyPBI.
中文翻译:

烷基化聚苯并咪唑的碱性阴离子交换膜
尽管最近有大量关于碱性阴离子交换膜 (AAEM) 的文献报道,但尚未充分解决的主要瓶颈是开发具有优异碱性稳定性和高氢氧化物电导率的聚合物膜。为了减轻这些,在这项工作中,我们研究了产生离子的烷基结构对改善具有双离子交换位点(即吡啶鎓和咪唑鎓)的 AAEM 的碱稳定性和氢氧化物电导率的影响。三种不同类型的聚合物,聚苯并咪唑 (PBI)、吡啶桥连 PBI (PyPBI) 和叔丁基 PyPBI (t But-PyPBI),通过咪唑环的烷基化,通过与各种烷基碘如甲基碘、丁基碘和异丁基碘反应,转化为它们的碘化物形式。聚苯并咪唑鎓膜的所有碘化物形式都转化为氢氧化物形式,从而通过将它们浸入 KOH 溶液中来获得 AAEM。FT-IR 和1H NMR 光谱研究用于确认此处研究的所有三种 PBI 结构的聚合物、碘化物形式和负载 KOH 的 AAEM 的结构。研究了所有膜的离子交换容量 (IEC)、氢氧化物电导率以及热、机械和碱稳定性,我们发现用丁基碘 (PyPBI-BuI) 烷基化的 PyPBI 具有最高的 IEC,3.37 mequiv/g,和在这项工作中开发的所有膜中,在 80°C 时,氢氧化物的最大电导率为 128.6 mS/cm。所有膜,无论聚合物结构如何,当用异丁基和丁基链烷基化时,即使在高达 60°C 的 5 M KOH 水溶液中也显示出优异的碱稳定性,而当用碘甲烷烷基化时,所有膜即使在 1 M KOH 在室温下。该观察结果表明烷基化部分结构对 AAEM 的碱性稳定性的重要性。这种显着的稳定性可能是由于烷基部分的庞大性质,这阻止了氢氧根离子对吡啶鎓和咪唑鎓基团的攻击。此外,我们使用 DFT 计算的计算研究证实,在较长(异丁基、丁基)烷基化链的情况下,尤其是在 PyPBI 和 t但是-PyPBI。
更新日期:2021-09-27
中文翻译:

烷基化聚苯并咪唑的碱性阴离子交换膜
尽管最近有大量关于碱性阴离子交换膜 (AAEM) 的文献报道,但尚未充分解决的主要瓶颈是开发具有优异碱性稳定性和高氢氧化物电导率的聚合物膜。为了减轻这些,在这项工作中,我们研究了产生离子的烷基结构对改善具有双离子交换位点(即吡啶鎓和咪唑鎓)的 AAEM 的碱稳定性和氢氧化物电导率的影响。三种不同类型的聚合物,聚苯并咪唑 (PBI)、吡啶桥连 PBI (PyPBI) 和叔丁基 PyPBI (t But-PyPBI),通过咪唑环的烷基化,通过与各种烷基碘如甲基碘、丁基碘和异丁基碘反应,转化为它们的碘化物形式。聚苯并咪唑鎓膜的所有碘化物形式都转化为氢氧化物形式,从而通过将它们浸入 KOH 溶液中来获得 AAEM。FT-IR 和1H NMR 光谱研究用于确认此处研究的所有三种 PBI 结构的聚合物、碘化物形式和负载 KOH 的 AAEM 的结构。研究了所有膜的离子交换容量 (IEC)、氢氧化物电导率以及热、机械和碱稳定性,我们发现用丁基碘 (PyPBI-BuI) 烷基化的 PyPBI 具有最高的 IEC,3.37 mequiv/g,和在这项工作中开发的所有膜中,在 80°C 时,氢氧化物的最大电导率为 128.6 mS/cm。所有膜,无论聚合物结构如何,当用异丁基和丁基链烷基化时,即使在高达 60°C 的 5 M KOH 水溶液中也显示出优异的碱稳定性,而当用碘甲烷烷基化时,所有膜即使在 1 M KOH 在室温下。该观察结果表明烷基化部分结构对 AAEM 的碱性稳定性的重要性。这种显着的稳定性可能是由于烷基部分的庞大性质,这阻止了氢氧根离子对吡啶鎓和咪唑鎓基团的攻击。此外,我们使用 DFT 计算的计算研究证实,在较长(异丁基、丁基)烷基化链的情况下,尤其是在 PyPBI 和 t但是-PyPBI。











































 京公网安备 11010802027423号
京公网安备 11010802027423号