当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly Facile, Regio- and Stereoselective Synthesis of Spiropyrrolidine-5-aza-2-oxindole Derivatives through Multicomponent 1,3-Dipolar Cycloaddition Reaction and Their In-Vitro and In-Silico Biological Studies
ChemistrySelect ( IF 1.9 ) Pub Date : 2021-09-15 , DOI: 10.1002/slct.202102118 P. P. Shinoj Kumar 1 , G. Krishnaswamy 2 , Nivedita R. Desai 1 , S. Sreenivasa 3 , D. B. Aruna Kumar 1, 2
ChemistrySelect ( IF 1.9 ) Pub Date : 2021-09-15 , DOI: 10.1002/slct.202102118 P. P. Shinoj Kumar 1 , G. Krishnaswamy 2 , Nivedita R. Desai 1 , S. Sreenivasa 3 , D. B. Aruna Kumar 1, 2
Affiliation
|
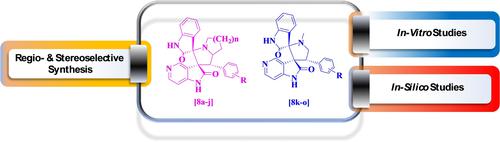
Highly facile one pot synthesis of an assortment of novel spiropyrrolidine 5-aza-2-oxindole derivatives via 1, 3-di polar cycloaddition reaction has been reported. The underlying concept of Huisgen reaction was applied wherein the azomethine ylides (1,3-dipoles), generated in-situ from isatin-derived compounds and cyclic / acyclic α-amino acids through the thermal decarboxylation, reacted with exo-cyclic benzylidine derivatives (dipolarophiles) of 5-aza-2-oxindole afforded plethora of spiropyrrolidine 5-aza-2-oxindoles in excellent yield. High regio- and stereo selectivity were accomplished employing this methodology and optimum yield was obtained when reaction carried out under methanol reflux in presence of triethylamine. Furthermore, the compounds were tested for their in-vitro biological potency as anti-microbial and anti-mycobacterial. Antibacterial screening result reveals that out of fifteen compounds four compounds emerged with moderate to reasonable activity against gram negative E. coli and P. aeruginosa. Antifungal screening resulted in identification of two compounds as moderate antifungal agents against A. niger. Antimycobacterial activity results revealed that the four compounds displayed good to moderate in-vitro activity with MIC=12.5 μg/mL against H37Rv strain as compared to standard pyrazinamide (MIC=3.12 μg/mL). Additionally, molecular docking study was carried out to understand the possible binding modes of the synthesized derivatives within the active site of antibacterial (PDB ID : 3ACX), antifungal (PDB ID : 1IYL) and antimycobacterial (PDB ID : 4TZT) target proteins and were found to display good docking energies. Besides, ADMET properties of the synthesized compounds have shown drug likeness with good oral absorption and moderate BBB permeability.
中文翻译:

Spiropyrrolidine-5-aza-2-oxindole 衍生物通过多组分 1,3-偶极环加成反应的高度简便、区域和立体选择性合成及其体外和计算机生物学研究
已经报道了通过 1, 3-双极性环加成反应非常容易地一锅合成各种新型螺吡咯烷 5-氮杂-2-羟吲哚衍生物。胡伊斯根反应的底层概念应用于其中所述甲亚胺叶立德(1,3-偶极子),生成的原位自靛红衍生的化合物和环状/非环状α氨基通过热脱羧的酸,与反应外切-环状亚苄基衍生物( 5-aza-2-oxindole 的双极亲和物)以极好的收率提供了过多的螺吡咯烷 5-aza-2-oxindole。使用该方法实现了高区域和立体选择性,并且在三乙胺存在下在甲醇回流下进行反应时获得了最佳产率。此外,还测试了这些化合物的作为抗微生物和抗分枝杆菌的体外生物效力。抗菌筛选结果表明,在十五种化合物中,有四种化合物对革兰氏阴性大肠杆菌和铜绿假单胞菌具有中等至合理的活性。抗真菌筛选导致鉴定出两种化合物是针对黑曲霉的中度抗真菌剂。抗分支杆菌活性的结果表明,四种化合物显示出良好的,中度的体外具有活性的MIC = 12.5 微克/毫升抗杆菌H37Rv菌株的相比于标准吡嗪酰胺(MIC = 3.12 微克/毫升)。此外,还进行了分子对接研究以了解合成衍生物在抗菌(PDB ID:3ACX)、抗真菌(PDB ID:1IYL)和抗分枝杆菌(PDB ID:4TZT)靶蛋白活性位点内的可能结合模式,并且发现显示出良好的对接能量。此外,合成化合物的 ADMET 特性显示出具有良好口服吸收和中等 BBB 渗透性的药物相似性。
更新日期:2021-09-15
中文翻译:

Spiropyrrolidine-5-aza-2-oxindole 衍生物通过多组分 1,3-偶极环加成反应的高度简便、区域和立体选择性合成及其体外和计算机生物学研究
已经报道了通过 1, 3-双极性环加成反应非常容易地一锅合成各种新型螺吡咯烷 5-氮杂-2-羟吲哚衍生物。胡伊斯根反应的底层概念应用于其中所述甲亚胺叶立德(1,3-偶极子),生成的原位自靛红衍生的化合物和环状/非环状α氨基通过热脱羧的酸,与反应外切-环状亚苄基衍生物( 5-aza-2-oxindole 的双极亲和物)以极好的收率提供了过多的螺吡咯烷 5-aza-2-oxindole。使用该方法实现了高区域和立体选择性,并且在三乙胺存在下在甲醇回流下进行反应时获得了最佳产率。此外,还测试了这些化合物的作为抗微生物和抗分枝杆菌的体外生物效力。抗菌筛选结果表明,在十五种化合物中,有四种化合物对革兰氏阴性大肠杆菌和铜绿假单胞菌具有中等至合理的活性。抗真菌筛选导致鉴定出两种化合物是针对黑曲霉的中度抗真菌剂。抗分支杆菌活性的结果表明,四种化合物显示出良好的,中度的体外具有活性的MIC = 12.5 微克/毫升抗杆菌H37Rv菌株的相比于标准吡嗪酰胺(MIC = 3.12 微克/毫升)。此外,还进行了分子对接研究以了解合成衍生物在抗菌(PDB ID:3ACX)、抗真菌(PDB ID:1IYL)和抗分枝杆菌(PDB ID:4TZT)靶蛋白活性位点内的可能结合模式,并且发现显示出良好的对接能量。此外,合成化合物的 ADMET 特性显示出具有良好口服吸收和中等 BBB 渗透性的药物相似性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号