当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly Diastereo- and Enantioselective Aza-Mannich Addition of Oxazolones to N-Boc Protected α-Amido Sulfones Catalyzed by Bifunctional Thiourea-modified Cinchona Alkaloid
ChemistrySelect ( IF 1.9 ) Pub Date : 2021-09-15 , DOI: 10.1002/slct.202102496 Hui Zhang 1, 2 , Lihua Song 1 , Weicheng Yuan 3 , Xiaomei Zhang 3
ChemistrySelect ( IF 1.9 ) Pub Date : 2021-09-15 , DOI: 10.1002/slct.202102496 Hui Zhang 1, 2 , Lihua Song 1 , Weicheng Yuan 3 , Xiaomei Zhang 3
Affiliation
|
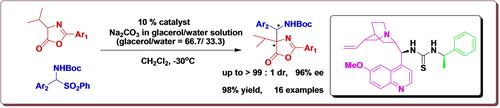
A diastereo- and enantioselective aza-Mannich addition of oxazolones to α-amino sulfones catalyzed by tertiary amine-1-phenylethyl thiourea based on cinchona structure has been developed. The reaction afforded the azlactone adducts bearing quaternary and tertiary stereogenic centers with high yields (up to 98 %) and moderate to excellent diastereo- and enantioselectivities (up to >99 : 1 dr and 96 % ee). The absolute configuration of a product was assigned by X-ray crystal structural analyses and a plausible reaction mechanism was proposed.
中文翻译:

双功能硫脲修饰的金鸡纳生物碱催化恶唑酮与 N-Boc 保护的 α-氨基砜的高度非对映选择性和对映选择性氮杂-曼尼希加成反应
基于金鸡纳结构的叔胺-1-苯乙基硫脲催化恶唑酮与α-氨基砜的非对映选择性和对映选择性氮杂-曼尼希加成反应已被开发。该反应以高产率(高达 98%)和中等至极好的非对映选择性和对映选择性(高达 >99:1 dr 和 96% ee)提供带有四级和三级立体中心的吖内酯加合物。通过 X 射线晶体结构分析确定了产物的绝对构型,并提出了合理的反应机制。
更新日期:2021-09-15
中文翻译:

双功能硫脲修饰的金鸡纳生物碱催化恶唑酮与 N-Boc 保护的 α-氨基砜的高度非对映选择性和对映选择性氮杂-曼尼希加成反应
基于金鸡纳结构的叔胺-1-苯乙基硫脲催化恶唑酮与α-氨基砜的非对映选择性和对映选择性氮杂-曼尼希加成反应已被开发。该反应以高产率(高达 98%)和中等至极好的非对映选择性和对映选择性(高达 >99:1 dr 和 96% ee)提供带有四级和三级立体中心的吖内酯加合物。通过 X 射线晶体结构分析确定了产物的绝对构型,并提出了合理的反应机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号