当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrazones in anion transporters: the detrimental effect of a second binding site
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-09-08 , DOI: 10.1039/d1ob01279g Luis Martínez-Crespo 1 , Lau Halgreen 1 , Márcio Soares 2 , Igor Marques 2 , Vítor Félix 2 , Hennie Valkenier 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-09-08 , DOI: 10.1039/d1ob01279g Luis Martínez-Crespo 1 , Lau Halgreen 1 , Márcio Soares 2 , Igor Marques 2 , Vítor Félix 2 , Hennie Valkenier 1
Affiliation
|
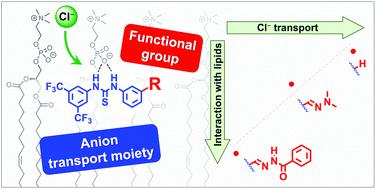
Synthetic anion transporters can be developed using anion receptors that are able to bind the anion and stabilize it in the lipophilic interior of a bilayer membrane, and they usually contain functional groups with acidic NHs, such as ureas, thioureas and squaramides. To assess the suitability of acylhydrazones as a new functional group for the preparation of anion transporters, we have studied a family of thioureas functionalized with these and related functional groups. 1H NMR titrations and DFT calculations indicate that the thioureas bearing acylhydrazone groups behave as chloride receptors with two separate binding sites, of which the acylhydrazone binds weaker than the thiourea. Chloride transport studies show that the additional binding site has a detrimental effect on thiourea-based transporters, and this phenomenon is also observed for bis(thio)ureas with two separate binding sites. We propose that the presence of a second anion binding unit hinders the transport activity of the thiourea due to additional interactions with the phospholipids of the membrane. In agreement with this hypothesis, extensive molecular dynamics simulations suggest that the molecules will tend to be positioned in the water/lipid interface, driven by the interaction of the NHs of the thiourea and of the acylhydrazone groups with the POPC polar head groups and water molecules. Moreover, the interaction energies show that the poorest transporters have indeed the strongest interactions with the membrane phospholipids, inhibiting chloride transport. This detrimental effect of additional functional groups on transport activity should be considered when designing new ion transporters, unless these groups cooperatively promote anion recognition and transmembrane transport.
中文翻译:

阴离子转运蛋白中的腙:第二个结合位点的不利影响
可以使用能够结合阴离子并将其稳定在双层膜的亲脂内部的阴离子受体来开发合成的阴离子转运蛋白,它们通常包含带有酸性 NH 的官能团,例如脲、硫脲和方酰胺。为了评估酰基腙作为制备阴离子转运蛋白的新官能团的适用性,我们研究了用这些和相关官能团官能化的硫脲家族。1H NMR滴定和DFT计算表明,带有酰基腙基团的硫脲表现为具有两个独立结合位点的氯受体,其中酰基腙的结合比硫脲弱。氯化物转运研究表明,额外的结合位点对基于硫脲的转运蛋白具有不利影响,对于具有两个独立结合位点的双(硫)脲也观察到这种现象。我们提出第二个阴离子结合单元的存在会阻碍硫脲的转运活性,因为它与膜的磷脂有额外的相互作用。与这一假设一致,广泛的分子动力学模拟表明分子将倾向于定位在水/脂质界面中,由硫脲的NH和酰基腙基团与POPC极性头基和水分子的相互作用驱动。此外,相互作用能表明,最差的转运蛋白确实与膜磷脂有最强的相互作用,抑制了氯离子的转运。在设计新的离子转运蛋白时,应考虑额外官能团对转运活性的不利影响,除非这些官能团协同促进阴离子识别和跨膜转运。
更新日期:2021-09-15
中文翻译:

阴离子转运蛋白中的腙:第二个结合位点的不利影响
可以使用能够结合阴离子并将其稳定在双层膜的亲脂内部的阴离子受体来开发合成的阴离子转运蛋白,它们通常包含带有酸性 NH 的官能团,例如脲、硫脲和方酰胺。为了评估酰基腙作为制备阴离子转运蛋白的新官能团的适用性,我们研究了用这些和相关官能团官能化的硫脲家族。1H NMR滴定和DFT计算表明,带有酰基腙基团的硫脲表现为具有两个独立结合位点的氯受体,其中酰基腙的结合比硫脲弱。氯化物转运研究表明,额外的结合位点对基于硫脲的转运蛋白具有不利影响,对于具有两个独立结合位点的双(硫)脲也观察到这种现象。我们提出第二个阴离子结合单元的存在会阻碍硫脲的转运活性,因为它与膜的磷脂有额外的相互作用。与这一假设一致,广泛的分子动力学模拟表明分子将倾向于定位在水/脂质界面中,由硫脲的NH和酰基腙基团与POPC极性头基和水分子的相互作用驱动。此外,相互作用能表明,最差的转运蛋白确实与膜磷脂有最强的相互作用,抑制了氯离子的转运。在设计新的离子转运蛋白时,应考虑额外官能团对转运活性的不利影响,除非这些官能团协同促进阴离子识别和跨膜转运。











































 京公网安备 11010802027423号
京公网安备 11010802027423号