当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lewis Superacidic Catecholato Phosphonium Ions: Phosphorus–Ligand Cooperative C–H Bond Activation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-09-15 , DOI: 10.1021/jacs.1c07905 Daniel Roth 1 , Judith Stirn 1, 2 , Douglas W Stephan 2 , Lutz Greb 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-09-15 , DOI: 10.1021/jacs.1c07905 Daniel Roth 1 , Judith Stirn 1, 2 , Douglas W Stephan 2 , Lutz Greb 1
Affiliation
|
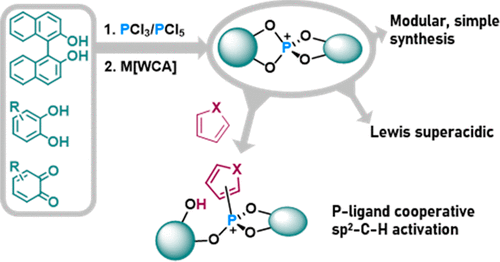
A series of catecholato phosphonium ions, including the first stable bis(catecholato)-substituted derivatives, are isolated and fully characterized. The cations rank among the most potent literature-known Lewis acids on the Gutmann–Beckett and ion affinity scales. In contrast to halogenated or multiply charged phosphorus cations, Lewis superacidity is imparted by structural constraints, as disclosed by energy decomposition analysis. The modular access provides a tunable scaffold while maintaining extreme affinity, demonstrated by the synthesis of a chiral Lewis superacid. The combination of electrophilic phosphorus and basic oxygen substituents leverages new reactivity modes by phosphorus–ligand cooperativity. With this, a phosphorus-mediated C–H bond activation is accomplished.
中文翻译:

Lewis 超酸性儿茶酚合膦离子:磷-配体协同 C-H 键活化
一系列儿茶酚鏻离子,包括第一个稳定的双(儿茶酚)取代衍生物,被分离出来并得到充分表征。这些阳离子在 Gutmann-Beckett 和离子亲和性尺度上属于最有效的文献中已知的路易斯酸。与卤化或多电荷磷阳离子相比,路易斯超酸性是由结构约束赋予的,如能量分解分析所揭示的。模块化通路提供了一个可调节的支架,同时保持了极高的亲和力,手性路易斯超强酸的合成证明了这一点。亲电磷和碱性氧取代基的组合通过磷-配体协同作用利用了新的反应模式。这样,就完成了磷介导的 C-H 键活化。
更新日期:2021-09-29
中文翻译:

Lewis 超酸性儿茶酚合膦离子:磷-配体协同 C-H 键活化
一系列儿茶酚鏻离子,包括第一个稳定的双(儿茶酚)取代衍生物,被分离出来并得到充分表征。这些阳离子在 Gutmann-Beckett 和离子亲和性尺度上属于最有效的文献中已知的路易斯酸。与卤化或多电荷磷阳离子相比,路易斯超酸性是由结构约束赋予的,如能量分解分析所揭示的。模块化通路提供了一个可调节的支架,同时保持了极高的亲和力,手性路易斯超强酸的合成证明了这一点。亲电磷和碱性氧取代基的组合通过磷-配体协同作用利用了新的反应模式。这样,就完成了磷介导的 C-H 键活化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号