当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Power of the Proton: From Superacidic Media to Superelectrophile Catalysis
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-09-14 , DOI: 10.1021/jacs.1c07614 Hendrik F T Klare 1 , Martin Oestreich 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-09-14 , DOI: 10.1021/jacs.1c07614 Hendrik F T Klare 1 , Martin Oestreich 1
Affiliation
|
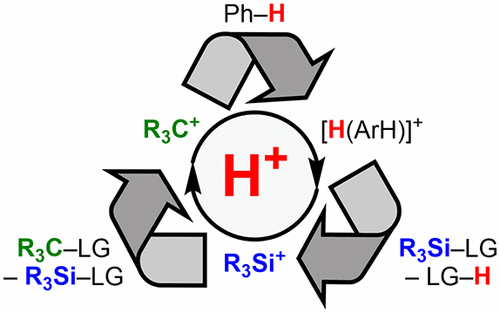
Superacidic media became famous in connection with carbocations. Yet not all reactive intermediates can be generated, characterized, and eventually isolated from these Brønsted acid/Lewis acid cocktails. The counteranion, that is the conjugate base, in these systems is often too nucleophilic and/or engages in redox chemistry with the newly formed cation. The Brønsted acidity, especially superacidity, is in fact often not even crucial unless protonation of extremely weak bases needs to be achieved. Instead, it is the chemical robustness of the aforementioned counteranion that determines the success of the protolysis. The advent of molecular Brønsted superacids derived from weakly coordinating, redox-inactive counteranions that do withstand the enormous reactivity of superelectrophiles such as silicon cations completely changed the whole field. This Perspective summarizes general aspects of medium and molecular Brønsted acidity and shows how applications of molecular Brønsted superacids have advanced from stoichiometric reactions to catalytic processes involving protons and in situ generated superelectrophiles.
中文翻译:

质子的力量:从超酸性介质到超亲电催化
超酸性介质因与碳正离子有关而闻名。然而,并非所有反应性中间体都可以从这些布朗斯台德酸/路易斯酸混合物中生成、表征并最终分离。这些系统中的抗衡阴离子,即共轭碱,通常过于亲核和/或与新形成的阳离子发生氧化还原化学反应。布朗斯台德酸度,尤其是超酸度,实际上通常甚至不重要,除非需要实现极弱碱的质子化。相反,是上述抗衡阴离子的化学稳定性决定了质子分解的成功。分子式的出现Brønsted 超强酸源自弱配位、氧化还原不活跃的反阴离子,这些反阴离子确实能够承受超亲电体(如硅阳离子)的巨大反应性,彻底改变了整个领域。该观点总结了中等和分子布朗斯台德酸度的一般方面,并展示了分子布朗斯台德超强酸的应用如何从化学计量反应发展到涉及质子和原位产生的超亲电体的催化过程。
更新日期:2021-09-29
中文翻译:

质子的力量:从超酸性介质到超亲电催化
超酸性介质因与碳正离子有关而闻名。然而,并非所有反应性中间体都可以从这些布朗斯台德酸/路易斯酸混合物中生成、表征并最终分离。这些系统中的抗衡阴离子,即共轭碱,通常过于亲核和/或与新形成的阳离子发生氧化还原化学反应。布朗斯台德酸度,尤其是超酸度,实际上通常甚至不重要,除非需要实现极弱碱的质子化。相反,是上述抗衡阴离子的化学稳定性决定了质子分解的成功。分子式的出现Brønsted 超强酸源自弱配位、氧化还原不活跃的反阴离子,这些反阴离子确实能够承受超亲电体(如硅阳离子)的巨大反应性,彻底改变了整个领域。该观点总结了中等和分子布朗斯台德酸度的一般方面,并展示了分子布朗斯台德超强酸的应用如何从化学计量反应发展到涉及质子和原位产生的超亲电体的催化过程。









































 京公网安备 11010802027423号
京公网安备 11010802027423号