当前位置:
X-MOL 学术
›
J. Chem. Theory Comput.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Relative Affinities of Protein–Cholesterol Interactions from Equilibrium Molecular Dynamics Simulations
Journal of Chemical Theory and Computation ( IF 5.7 ) Pub Date : 2021-09-15 , DOI: 10.1021/acs.jctc.1c00547 T Bertie Ansell 1 , Luke Curran 1 , Michael R Horrell 1 , Tanadet Pipatpolkai 1, 2 , Suzanne C Letham 1, 3 , Wanling Song 1 , Christian Siebold 4 , Phillip J Stansfeld 5 , Mark S P Sansom 1 , Robin A Corey 1
Journal of Chemical Theory and Computation ( IF 5.7 ) Pub Date : 2021-09-15 , DOI: 10.1021/acs.jctc.1c00547 T Bertie Ansell 1 , Luke Curran 1 , Michael R Horrell 1 , Tanadet Pipatpolkai 1, 2 , Suzanne C Letham 1, 3 , Wanling Song 1 , Christian Siebold 4 , Phillip J Stansfeld 5 , Mark S P Sansom 1 , Robin A Corey 1
Affiliation
|
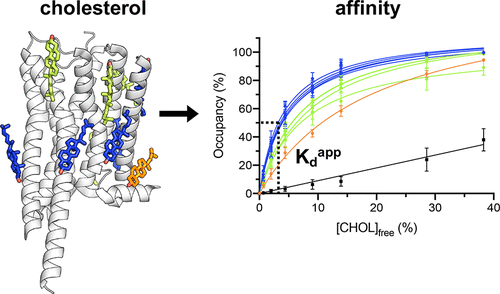
Specific interactions of lipids with membrane proteins contribute to protein stability and function. Multiple lipid interactions surrounding a membrane protein are often identified in molecular dynamics (MD) simulations and are, increasingly, resolved in cryo-electron microscopy (cryo-EM) densities. Determining the relative importance of specific interaction sites is aided by determination of lipid binding affinities using experimental or simulation methods. Here, we develop a method for determining protein–lipid binding affinities from equilibrium coarse-grained MD simulations using binding saturation curves, designed to mimic experimental protocols. We apply this method to directly obtain affinities for cholesterol binding to multiple sites on a range of membrane proteins and compare our results with free energies obtained from density-based equilibrium methods and with potential of mean force calculations, getting good agreement with respect to the ranking of affinities for different sites. Thus, our binding saturation method provides a robust, high-throughput alternative for determining the relative consequence of individual sites seen in, e.g., cryo-EM derived membrane protein structures surrounded by an array of ancillary lipid densities.
中文翻译:

平衡分子动力学模拟中蛋白质-胆固醇相互作用的相对亲和力
脂质与膜蛋白的特定相互作用有助于蛋白质的稳定性和功能。膜蛋白周围的多种脂质相互作用通常在分子动力学 (MD) 模拟中被识别,并且越来越多地在冷冻电子显微镜 (cryo-EM) 密度中得到解决。使用实验或模拟方法确定脂质结合亲和力有助于确定特定相互作用位点的相对重要性。在这里,我们开发了一种使用结合饱和曲线从平衡粗粒度 MD 模拟中确定蛋白质-脂质结合亲和力的方法,旨在模拟实验方案。我们应用这种方法直接获得胆固醇与一系列膜蛋白上多个位点结合的亲和力,并将我们的结果与从基于密度的平衡方法获得的自由能以及平均力计算的潜力进行比较,在排名方面获得了良好的一致性不同站点的亲和力。因此,我们的结合饱和方法提供了一种稳健的、高通量的替代方案,用于确定在例如被一系列辅助脂质密度包围的冷冻电镜衍生的膜蛋白结构中看到的各个位点的相对结果。
更新日期:2021-10-12
中文翻译:

平衡分子动力学模拟中蛋白质-胆固醇相互作用的相对亲和力
脂质与膜蛋白的特定相互作用有助于蛋白质的稳定性和功能。膜蛋白周围的多种脂质相互作用通常在分子动力学 (MD) 模拟中被识别,并且越来越多地在冷冻电子显微镜 (cryo-EM) 密度中得到解决。使用实验或模拟方法确定脂质结合亲和力有助于确定特定相互作用位点的相对重要性。在这里,我们开发了一种使用结合饱和曲线从平衡粗粒度 MD 模拟中确定蛋白质-脂质结合亲和力的方法,旨在模拟实验方案。我们应用这种方法直接获得胆固醇与一系列膜蛋白上多个位点结合的亲和力,并将我们的结果与从基于密度的平衡方法获得的自由能以及平均力计算的潜力进行比较,在排名方面获得了良好的一致性不同站点的亲和力。因此,我们的结合饱和方法提供了一种稳健的、高通量的替代方案,用于确定在例如被一系列辅助脂质密度包围的冷冻电镜衍生的膜蛋白结构中看到的各个位点的相对结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号