当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Cu(I)/Mo(VI) Reactivity of a Bifunctional Heterodinucleating Ligand on a Xanthene Platform
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-09-14 , DOI: 10.1021/acs.inorgchem.1c01735 Umesh I Kaluarachchige Don 1 , Sudheer S Kurup 1 , Thilini S Hollingsworth 1 , Cassandra L Ward 2 , Richard L Lord 3 , Stanislav Groysman 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-09-14 , DOI: 10.1021/acs.inorgchem.1c01735 Umesh I Kaluarachchige Don 1 , Sudheer S Kurup 1 , Thilini S Hollingsworth 1 , Cassandra L Ward 2 , Richard L Lord 3 , Stanislav Groysman 1
Affiliation
|
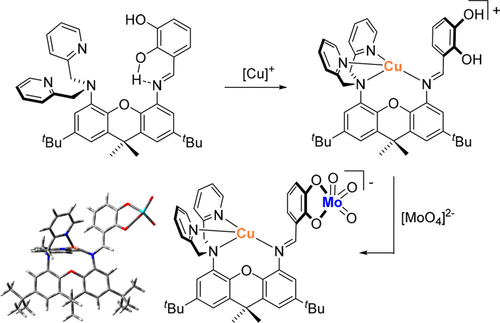
In an effort to probe the feasibility of a model of Mo-Cu CODH (CODH = carbon monoxide dehydrogenase) lacking a bridging sulfido group, the new heterodinucleating ligand LH2 was designed and its Cu(I)/Mo(VI) reactivity was investigated. LH2 ((E)-3-(((5-(bis(pyridin-2-ylmethyl)amino)-2,7-di-tert-butyl-9,9-dimethyl-9H-xanthen-4-yl)imino)methyl)benzene-1,2-diol) features two different chelating positions bridged by a xanthene linker: bis(pyridyl)amine for Cu(I) and catecholate for Mo(VI). LH2 was synthesized via the initial protection of one of the amine positions, followed by two consecutive alkylations of the second position, deprotection, and condensation to attach the catechol functionality. LH2 was found to exhibit dynamic cooperativity between two reactive sites mediated by H-bonding of the catechol protons. In the free ligand, catechol protons exhibit H-bonding with imine (intramolecular) and with pyridine (intermolecular in the solid state). The reaction of LH2 with [Cu(NCMe)4]+ led to the tetradentate coordination of Cu(I) via all nitrogen donors of the ligand, including the imine. Cu(I) complexes were characterized by multinuclear NMR spectroscopy, high-resolution mass spectrometry (HRMS), X-ray crystallography, and DFT calculations. Cu(I) coordination to the imine disrupted H-bonding and caused rotation away from the catechol arm. The reaction of the Cu(I) complex [Cu(LH2)]+ with a variety of monodentate ligands X (PPh3, Cl–, SCN–, CN–) released the metal from coordination to the imine, thereby restoring imine H-bonding with the catechol proton. The second catechol proton engages in H-bonding with Cu–X (X = Cl, CN, SCN), which can be intermolecular (XRD) or intramolecular (DFT). The reaction of LH2 with molybdate [MoO4]2– led to incorporation of [MoVIO3] at the catecholate position, producing [MoO3(L)]2–. Similarly, the reaction of [Cu(LH2)]+ with [MoO4]2– formed the heterodinuclear complex [CuMoO3(L)]−. Both complexes were characterized by multinuclear NMR, UV–vis, and HRMS. HRMS in both cases confirmed the constitution of the complexes, containing molecular ions with the expected isotopic distribution.
中文翻译:

双功能杂双成核配体在呫吨平台上的合成和 Cu(I)/Mo(VI) 反应性
为了探索缺乏桥接硫基的 Mo-Cu CODH(CODH = 一氧化碳脱氢酶)模型的可行性,设计了新的异双核配体 LH 2并研究了其 Cu(I)/Mo(VI) 反应性. LH 2 (( E )-3-((((5-(bis(pyridin-2-ylmethyl)amino)-2,7-di- tert -butyl-9,9-dimethyl-9 H -xanthen-4-yl) )亚氨基)甲基)苯-1,2-二醇)具有两个不同的螯合位置,由呫吨连接体桥接:双(吡啶基)胺用于 Cu(I)和儿茶酚酸酯用于 Mo(VI)。LH 2通过对胺位置之一进行初始保护,然后对第二个位置进行两次连续烷基化、脱保护和缩合以连接邻苯二酚官能团来合成。发现LH 2表现出由儿茶酚质子的氢键介导的两个反应位点之间的动态协同作用。在游离配体中,儿茶酚质子与亚胺(分子内)和吡啶(固态分子间)显示氢键。LH 2与[Cu(NCMe) 4 ] + 的反应通过配体的所有氮供体(包括亚胺)导致 Cu(I) 的四齿配位。Cu(I) 配合物的特征在于多核 NMR 光谱、高分辨率质谱 (HRMS)、X 射线晶体学和 DFT 计算。Cu(I) 与亚胺的配位破坏了 H 键合并导致旋转远离儿茶酚臂。Cu(I) 配合物 [Cu(LH 2 )] +与多种单齿配体 X (PPh 3 , Cl – , SCN – , CN –) 的反应) 释放金属与亚胺的配位,从而恢复亚胺与儿茶酚质子的氢键。第二个儿茶酚质子与 Cu-X(X = Cl、CN、SCN)发生氢键,可以是分子间(XRD)或分子内(DFT)。LH 2与钼酸盐[MoO 4 ] 2– 的反应导致[Mo VI O 3 ] 在儿茶酚酸盐位置结合,产生[MoO 3 (L)] 2–。类似地,[Cu(LH 2 )] +与[MoO 4 ] 2– 反应形成异双核配合物[CuMoO 3 (L)] -. 两种配合物均通过多核 NMR、UV-vis 和 HRMS 进行表征。两种情况下的 HRMS 都证实了复合物的构成,其中包含具有预期同位素分布的分子离子。
更新日期:2021-10-04
中文翻译:

双功能杂双成核配体在呫吨平台上的合成和 Cu(I)/Mo(VI) 反应性
为了探索缺乏桥接硫基的 Mo-Cu CODH(CODH = 一氧化碳脱氢酶)模型的可行性,设计了新的异双核配体 LH 2并研究了其 Cu(I)/Mo(VI) 反应性. LH 2 (( E )-3-((((5-(bis(pyridin-2-ylmethyl)amino)-2,7-di- tert -butyl-9,9-dimethyl-9 H -xanthen-4-yl) )亚氨基)甲基)苯-1,2-二醇)具有两个不同的螯合位置,由呫吨连接体桥接:双(吡啶基)胺用于 Cu(I)和儿茶酚酸酯用于 Mo(VI)。LH 2通过对胺位置之一进行初始保护,然后对第二个位置进行两次连续烷基化、脱保护和缩合以连接邻苯二酚官能团来合成。发现LH 2表现出由儿茶酚质子的氢键介导的两个反应位点之间的动态协同作用。在游离配体中,儿茶酚质子与亚胺(分子内)和吡啶(固态分子间)显示氢键。LH 2与[Cu(NCMe) 4 ] + 的反应通过配体的所有氮供体(包括亚胺)导致 Cu(I) 的四齿配位。Cu(I) 配合物的特征在于多核 NMR 光谱、高分辨率质谱 (HRMS)、X 射线晶体学和 DFT 计算。Cu(I) 与亚胺的配位破坏了 H 键合并导致旋转远离儿茶酚臂。Cu(I) 配合物 [Cu(LH 2 )] +与多种单齿配体 X (PPh 3 , Cl – , SCN – , CN –) 的反应) 释放金属与亚胺的配位,从而恢复亚胺与儿茶酚质子的氢键。第二个儿茶酚质子与 Cu-X(X = Cl、CN、SCN)发生氢键,可以是分子间(XRD)或分子内(DFT)。LH 2与钼酸盐[MoO 4 ] 2– 的反应导致[Mo VI O 3 ] 在儿茶酚酸盐位置结合,产生[MoO 3 (L)] 2–。类似地,[Cu(LH 2 )] +与[MoO 4 ] 2– 反应形成异双核配合物[CuMoO 3 (L)] -. 两种配合物均通过多核 NMR、UV-vis 和 HRMS 进行表征。两种情况下的 HRMS 都证实了复合物的构成,其中包含具有预期同位素分布的分子离子。










































 京公网安备 11010802027423号
京公网安备 11010802027423号