当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High-Temperature Stability and Pyrolysis Kinetics and Mechanism of Bio-Based and Petro-Based Resins Using TG–FTIR/MS
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2021-09-14 , DOI: 10.1021/acs.iecr.1c02535 Dan Zhou 1 , Xiaopeng Chen 1 , Jiezhen Liang 1 , Xiaojie Wei 1 , Chenghong Wu 1 , Wenhui Li 1 , Linlin Wang 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2021-09-14 , DOI: 10.1021/acs.iecr.1c02535 Dan Zhou 1 , Xiaopeng Chen 1 , Jiezhen Liang 1 , Xiaojie Wei 1 , Chenghong Wu 1 , Wenhui Li 1 , Linlin Wang 1
Affiliation
|
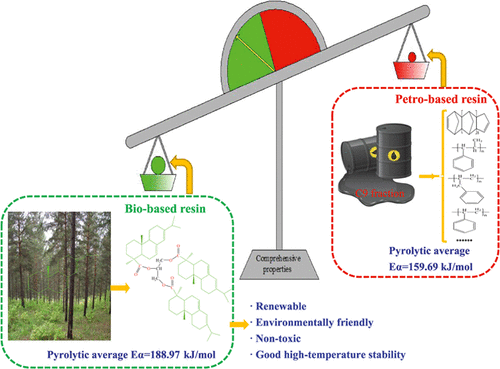
Pyrolysis behavior of resins is essential for their high-temperature application. Herein, the high-temperature stability and pyrolysis kinetics and mechanism of rosin glyceride (RGE), hydrogenated rosin glyceride (HRGE), C9 petro-based resin (C9PR), and hydrogenated C9 petro-based resin (HC9PR) under a nonoxidizing atmosphere were investigated by thermogravimetry coupled with Fourier transform infrared spectrometry or mass spectrometry (TG–FTIR/MS) techniques. Friedman and Starink methods as well as reaction-order and truncated Sestak–Berggren models were used to evaluate kinetic and thermodynamic parameters, and results indicated that f(α) = (1 – α)n was the most probable pyrolysis mechanism for different resins. In addition, the average activation energies for pyrolysis of RGE, HRGE, C9PR, and HC9PR obtained by the Starink method were 188.97, 170.95, 159.69, and 151.66 kJ/mol, respectively, suggesting that bio-based resins exhibited better high-temperature stability than cycloaliphatic or aromatic petro-based resins thanks to their unique tricyclic phenanthrene structures, and the high-temperature stability of resins mildly would decrease after hydromodification due to the cracking of saturated bonds, which was well supported by TG–FTIR/MS analyses. Possible pyrolysis pathways were proposed.
中文翻译:

使用 TG-FTIR/MS 研究生物基和石油基树脂的高温稳定性和热解动力学和机理
树脂的热解行为对其高温应用至关重要。在此,松香甘油酯(RGE)、氢化松香甘油酯(HRGE)、C9石油基树脂(C9PR)和氢化C9石油基树脂(HC9PR)在非氧化气氛下的高温稳定性和热解动力学和机理是通过热重分析结合傅里叶变换红外光谱或质谱 (TG-FTIR/MS) 技术进行研究。Friedman 和 Starink 方法以及反应级和截断的 Sestak-Berggren 模型用于评估动力学和热力学参数,结果表明f (α) = (1 – α) n是不同树脂最可能的热解机制。此外,通过 Starink 法获得的 RGE、HRGE、C9PR 和 HC9PR 热解的平均活化能分别为 188.97、170.95、159.69 和 151.66 kJ/mol,表明生物基树脂表现出更好的高温稳定性由于其独特的三环菲结构,它比脂环族或芳族石油基树脂的性能好,并且由于饱和键的裂解,树脂的高温稳定性在加氢改性后会轻微降低,TG-FTIR / MS分析很好地支持了这一点。提出了可能的热解途径。
更新日期:2021-09-29
中文翻译:

使用 TG-FTIR/MS 研究生物基和石油基树脂的高温稳定性和热解动力学和机理
树脂的热解行为对其高温应用至关重要。在此,松香甘油酯(RGE)、氢化松香甘油酯(HRGE)、C9石油基树脂(C9PR)和氢化C9石油基树脂(HC9PR)在非氧化气氛下的高温稳定性和热解动力学和机理是通过热重分析结合傅里叶变换红外光谱或质谱 (TG-FTIR/MS) 技术进行研究。Friedman 和 Starink 方法以及反应级和截断的 Sestak-Berggren 模型用于评估动力学和热力学参数,结果表明f (α) = (1 – α) n是不同树脂最可能的热解机制。此外,通过 Starink 法获得的 RGE、HRGE、C9PR 和 HC9PR 热解的平均活化能分别为 188.97、170.95、159.69 和 151.66 kJ/mol,表明生物基树脂表现出更好的高温稳定性由于其独特的三环菲结构,它比脂环族或芳族石油基树脂的性能好,并且由于饱和键的裂解,树脂的高温稳定性在加氢改性后会轻微降低,TG-FTIR / MS分析很好地支持了这一点。提出了可能的热解途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号