当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cellulosic Ternary Nanocomposite for Affordable and Sustainable Fluoride Removal
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2021-09-14 , DOI: 10.1021/acssuschemeng.1c03272 Moses Egor 1, 2, 3 , Avula Anil Kumar 3 , Tripti Ahuja 3 , Sritama Mukherjee 3 , Amrita Chakraborty 3 , Chennu Sudhakar 3 , Pillalamarri Srikrishnarka 3 , Sandeep Bose 3 , Swathy Jakka Ravindran 3 , Thalappil Pradeep 3
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2021-09-14 , DOI: 10.1021/acssuschemeng.1c03272 Moses Egor 1, 2, 3 , Avula Anil Kumar 3 , Tripti Ahuja 3 , Sritama Mukherjee 3 , Amrita Chakraborty 3 , Chennu Sudhakar 3 , Pillalamarri Srikrishnarka 3 , Sandeep Bose 3 , Swathy Jakka Ravindran 3 , Thalappil Pradeep 3
Affiliation
|
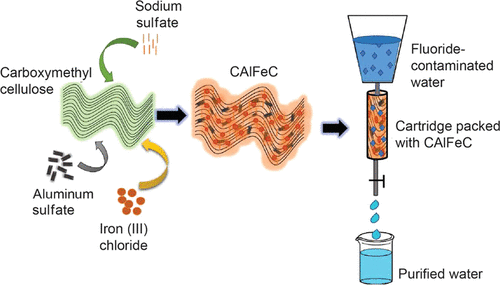
Adsorption is shown to be an extremely affordable and sustainable way of producing clean water, particularly in resource-limited settings. In this paper, we sought to synthesize an effective cellulose-based composite adsorbent from eco-friendly, earth-abundant, and consequently affordable ingredients at room temperature for fluoride removal from drinking water. We utilized the synergistic effect of various renewable materials and active sites of metal oxyhydroxides in developing an effective adsorbent, which is physically stable under the conditions of use. Nanoscale oxyhydroxides of aluminum and iron were scaffolded into a matrix of carboxymethyl cellulose (CMC) to form a nanocomposite adsorbent, which was prepared in water, eventually making a water-stable porous solid. This was used in batch and cartridge adsorption experiments for fluoride removal. The adsorbent surface before (in situ) and after fluoride uptake was characterized using various analytical techniques. The in situ composite exhibited a surface area of 134.3 m2/g with an amorphous solid structure with Al and Fe uniformly distributed in the cellulose matrix. From the batch adsorption experiments, we observed 80% fluoride removal within the first 3 min of contact, with a maximum uptake capacity of 75.2 mg/g as modeled by the Langmuir adsorption isotherm, better than most reported materials. The adsorbent effectively reduced F– levels in field water from 10 to 0.3 mg/L, less than 1.5 mg/L the World Health Organization upper limit for drinking water. Optimum F– removal was achieved between the pH of 4–9; however, the effectiveness of the adsorbent was reduced in the presence of competing ions in the order PO43– > SiO32– > CO32– > HCO3– > SO42–. A cartridge experiment demonstrated the applicability of the adsorbent in a domestic point-of-use water purifier for defluoridation. Sustainability metrics of the material were evaluated. Defluoridation using the material is estimated to cost $3.3 per 1000 L of treated water at the scale of community implementation projects.
中文翻译:

纤维素三元纳米复合材料用于经济实惠且可持续的除氟
吸附被证明是一种极其经济且可持续的生产清洁水的方式,特别是在资源有限的环境中。在本文中,我们试图在室温下从环境友好、地球丰富且价格合理的成分中合成一种有效的纤维素基复合吸附剂,用于去除饮用水中的氟化物。我们利用各种可再生材料和金属羟基氧化物活性位点的协同作用开发了一种在使用条件下物理稳定的有效吸附剂。铝和铁的纳米级羟基氧化物支架成羧甲基纤维素(CMC)基质,形成纳米复合吸附剂,在水中制备,最终制成水稳定的多孔固体。这用于批量和筒式吸附实验以去除氟化物。之前的吸附剂表面(原位)和氟化物吸收后使用各种分析技术进行表征。的原位复合表现出134.3米表面积2与Al和Fe的无定形固体结构均匀地分布在纤维素基质/克。从批量吸附实验中,我们观察到在接触的前 3 分钟内去除了 80% 的氟化物,根据 Langmuir 吸附等温线模拟的最大吸收量为 75.2 mg/g,优于大多数报道的材料。该吸附剂有效地将田间水中的F -水平从 10 毫克/升降至 0.3 毫克/升,低于世界卫生组织饮用水上限的 1.5 毫克/升。最佳 F –在 4-9 的 pH 值之间实现了去除;然而,当竞争离子存在时,吸附剂的效率会降低,顺序为 PO 4 3– > SiO 3 2– > CO 3 2– > HCO 3 – > SO 4 2–。滤芯实验证明了该吸附剂在家用净水器除氟中的适用性。评估了材料的可持续性指标。在社区实施项目的规模内,使用该材料进行除氟的成本估计为每 1000 升处理水 3.3 美元。
更新日期:2021-09-27
中文翻译:

纤维素三元纳米复合材料用于经济实惠且可持续的除氟
吸附被证明是一种极其经济且可持续的生产清洁水的方式,特别是在资源有限的环境中。在本文中,我们试图在室温下从环境友好、地球丰富且价格合理的成分中合成一种有效的纤维素基复合吸附剂,用于去除饮用水中的氟化物。我们利用各种可再生材料和金属羟基氧化物活性位点的协同作用开发了一种在使用条件下物理稳定的有效吸附剂。铝和铁的纳米级羟基氧化物支架成羧甲基纤维素(CMC)基质,形成纳米复合吸附剂,在水中制备,最终制成水稳定的多孔固体。这用于批量和筒式吸附实验以去除氟化物。之前的吸附剂表面(原位)和氟化物吸收后使用各种分析技术进行表征。的原位复合表现出134.3米表面积2与Al和Fe的无定形固体结构均匀地分布在纤维素基质/克。从批量吸附实验中,我们观察到在接触的前 3 分钟内去除了 80% 的氟化物,根据 Langmuir 吸附等温线模拟的最大吸收量为 75.2 mg/g,优于大多数报道的材料。该吸附剂有效地将田间水中的F -水平从 10 毫克/升降至 0.3 毫克/升,低于世界卫生组织饮用水上限的 1.5 毫克/升。最佳 F –在 4-9 的 pH 值之间实现了去除;然而,当竞争离子存在时,吸附剂的效率会降低,顺序为 PO 4 3– > SiO 3 2– > CO 3 2– > HCO 3 – > SO 4 2–。滤芯实验证明了该吸附剂在家用净水器除氟中的适用性。评估了材料的可持续性指标。在社区实施项目的规模内,使用该材料进行除氟的成本估计为每 1000 升处理水 3.3 美元。











































 京公网安备 11010802027423号
京公网安备 11010802027423号