当前位置:
X-MOL 学术
›
J. Cell. Physiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dynamic alterations of H3K4me3 and H3K27me3 at ADAM17 and Jagged-1 gene promoters cause an inflammatory switch of endothelial cells
Journal of Cellular Physiology ( IF 4.5 ) Pub Date : 2021-09-14 , DOI: 10.1002/jcp.30579 Yash T Katakia 1 , Niyati P Thakkar 1 , Sumukh Thakar 1 , Ashima Sakhuja 1 , Raghav Goyal 1 , Harshita Sharma 1 , Rakshita Dave 1 , Ayushi Mandloi 1 , Sayan Basu 1 , Ishan Nigam 1 , Bhanu V R Kuncharam 2 , Shibasish Chowdhury 1 , Syamantak Majumder 1
Journal of Cellular Physiology ( IF 4.5 ) Pub Date : 2021-09-14 , DOI: 10.1002/jcp.30579 Yash T Katakia 1 , Niyati P Thakkar 1 , Sumukh Thakar 1 , Ashima Sakhuja 1 , Raghav Goyal 1 , Harshita Sharma 1 , Rakshita Dave 1 , Ayushi Mandloi 1 , Sayan Basu 1 , Ishan Nigam 1 , Bhanu V R Kuncharam 2 , Shibasish Chowdhury 1 , Syamantak Majumder 1
Affiliation
|
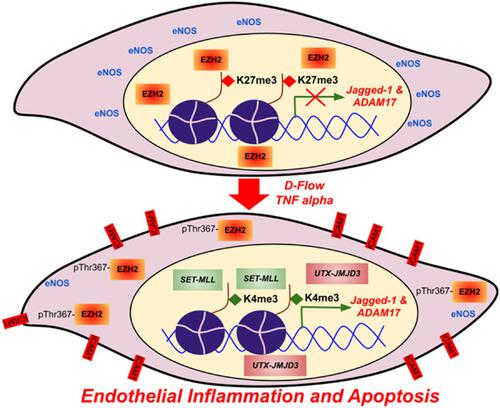
Histone protein modifications control the inflammatory state of many immune cells. However, how dynamic alteration in histone methylation causes endothelial inflammation and apoptosis is not clearly understood. To examine this, we explored two contrasting histone methylations; an activating histone H3 lysine 4 trimethylation (H3K4me3) and a repressive histone H3 lysine 27 trimethylation (H3K27me3) in endothelial cells (EC) undergoing inflammation. Through computer-aided reconstruction and 3D printing of the human coronary artery, we developed a unique model where EC were exposed to a pattern of oscillatory/disturbed flow as similar to in vivo conditions. Upon induction of endothelial inflammation, we detected a significant rise in H3K4me3 caused by an increase in the expression of SET1/COMPASS family of H3K4 methyltransferases, including MLL1, MLL2, and SET1B. In contrast, EC undergoing inflammation exhibited truncated H3K27me3 level engendered by EZH2 cytosolic translocation through threonine 367 phosphorylation and an increase in the expression of histone demethylating enzyme JMJD3 and UTX. Additionally, many SET1/COMPASS family of proteins, including MLL1 (C), MLL2, and WDR5, were associated with either UTX or JMJD3 or both and such association was elevated in EC upon exposure to inflammatory stimuli. Dynamic enrichment of H3K4me3 and loss of H3K27me3 at Notch-associated gene promoters caused ADAM17 and Jagged-1 derepression and abrupt Notch activation. Conversely, either reducing H3K4me3 or increasing H3K27me3 in EC undergoing inflammation attenuated Notch activation, endothelial inflammation, and apoptosis. Together, these findings indicate that dynamic chromatin modifications may cause an inflammatory and apoptotic switch of EC and that epigenetic reprogramming can potentially improve outcomes in endothelial inflammation-associated cardiovascular diseases.
中文翻译:

ADAM17 和 Jagged-1 基因启动子处 H3K4me3 和 H3K27me3 的动态改变导致内皮细胞的炎症转换
组蛋白修饰控制许多免疫细胞的炎症状态。然而,组蛋白甲基化的动态变化如何导致内皮炎症和细胞凋亡尚不清楚。为了检验这一点,我们探索了两种不同的组蛋白甲基化;在发生炎症的内皮细胞 (EC) 中激活组蛋白 H3 赖氨酸 4 三甲基化 (H3K4me3) 和抑制性组蛋白 H3 赖氨酸 27 三甲基化 (H3K27me3)。通过人类冠状动脉的计算机辅助重建和 3D 打印,我们开发了一种独特的模型,其中 EC 暴露于类似于体内条件的振荡/扰动流动模式。在诱导内皮炎症后,我们检测到 H3K4me3 的显着升高是由 H3K4 甲基转移酶的 SET1/COMPASS 家族(包括 MLL1)的表达增加引起的,MLL2 和 SET1B。相比之下,经历炎症的 EC 表现出由 EZH2 胞质易位通过苏氨酸 367 磷酸化和组蛋白去甲基化酶 JMJD3 和 UTX 的表达增加产生的截短的 H3K27me3 水平。此外,许多 SET1/COMPASS 蛋白家族,包括 MLL1 (C)、MLL2 和 WDR5,与 UTX 或 JMJD3 或两者相关,并且在暴露于炎症刺激后,这种关联在 EC 中升高。在 Notch 相关基因启动子处 H3K4me3 的动态富集和 H3K27me3 的丢失导致 许多 SET1/COMPASS 蛋白家族,包括 MLL1 (C)、MLL2 和 WDR5,与 UTX 或 JMJD3 或两者相关,并且在暴露于炎症刺激后,这种关联在 EC 中升高。在 Notch 相关基因启动子处 H3K4me3 的动态富集和 H3K27me3 的丢失导致 许多 SET1/COMPASS 蛋白家族,包括 MLL1 (C)、MLL2 和 WDR5,与 UTX 或 JMJD3 或两者相关,并且在暴露于炎症刺激后,这种关联在 EC 中升高。在 Notch 相关基因启动子处 H3K4me3 的动态富集和 H3K27me3 的丢失导致ADAM17和Jagged-1去抑制和突然的 Notch 激活。相反,在发生炎症的 EC 中减少 H3K4me3 或增加 H3K27me3 会减弱 Notch 激活、内皮炎症和细胞凋亡。总之,这些发现表明动态染色质修饰可能导致 EC 的炎症和凋亡转换,并且表观遗传重编程可能会改善内皮炎症相关心血管疾病的结果。
更新日期:2021-09-14
中文翻译:

ADAM17 和 Jagged-1 基因启动子处 H3K4me3 和 H3K27me3 的动态改变导致内皮细胞的炎症转换
组蛋白修饰控制许多免疫细胞的炎症状态。然而,组蛋白甲基化的动态变化如何导致内皮炎症和细胞凋亡尚不清楚。为了检验这一点,我们探索了两种不同的组蛋白甲基化;在发生炎症的内皮细胞 (EC) 中激活组蛋白 H3 赖氨酸 4 三甲基化 (H3K4me3) 和抑制性组蛋白 H3 赖氨酸 27 三甲基化 (H3K27me3)。通过人类冠状动脉的计算机辅助重建和 3D 打印,我们开发了一种独特的模型,其中 EC 暴露于类似于体内条件的振荡/扰动流动模式。在诱导内皮炎症后,我们检测到 H3K4me3 的显着升高是由 H3K4 甲基转移酶的 SET1/COMPASS 家族(包括 MLL1)的表达增加引起的,MLL2 和 SET1B。相比之下,经历炎症的 EC 表现出由 EZH2 胞质易位通过苏氨酸 367 磷酸化和组蛋白去甲基化酶 JMJD3 和 UTX 的表达增加产生的截短的 H3K27me3 水平。此外,许多 SET1/COMPASS 蛋白家族,包括 MLL1 (C)、MLL2 和 WDR5,与 UTX 或 JMJD3 或两者相关,并且在暴露于炎症刺激后,这种关联在 EC 中升高。在 Notch 相关基因启动子处 H3K4me3 的动态富集和 H3K27me3 的丢失导致 许多 SET1/COMPASS 蛋白家族,包括 MLL1 (C)、MLL2 和 WDR5,与 UTX 或 JMJD3 或两者相关,并且在暴露于炎症刺激后,这种关联在 EC 中升高。在 Notch 相关基因启动子处 H3K4me3 的动态富集和 H3K27me3 的丢失导致 许多 SET1/COMPASS 蛋白家族,包括 MLL1 (C)、MLL2 和 WDR5,与 UTX 或 JMJD3 或两者相关,并且在暴露于炎症刺激后,这种关联在 EC 中升高。在 Notch 相关基因启动子处 H3K4me3 的动态富集和 H3K27me3 的丢失导致ADAM17和Jagged-1去抑制和突然的 Notch 激活。相反,在发生炎症的 EC 中减少 H3K4me3 或增加 H3K27me3 会减弱 Notch 激活、内皮炎症和细胞凋亡。总之,这些发现表明动态染色质修饰可能导致 EC 的炎症和凋亡转换,并且表观遗传重编程可能会改善内皮炎症相关心血管疾病的结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号