当前位置:
X-MOL 学术
›
Appl. Organomet. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Study molecular modeling and the effect of some biological metals on the efficiency of norfloxacin in presence of 3-(bromoacetyl)coumarin
Applied Organometallic Chemistry ( IF 3.9 ) Pub Date : 2021-09-14 , DOI: 10.1002/aoc.6448 Walaa H. El‐Shwiniy 1, 2 , Manar A. Gamil 1 , Sadeek A. Sadeek 1 , Wael A. Zordok 1
Applied Organometallic Chemistry ( IF 3.9 ) Pub Date : 2021-09-14 , DOI: 10.1002/aoc.6448 Walaa H. El‐Shwiniy 1, 2 , Manar A. Gamil 1 , Sadeek A. Sadeek 1 , Wael A. Zordok 1
Affiliation
|
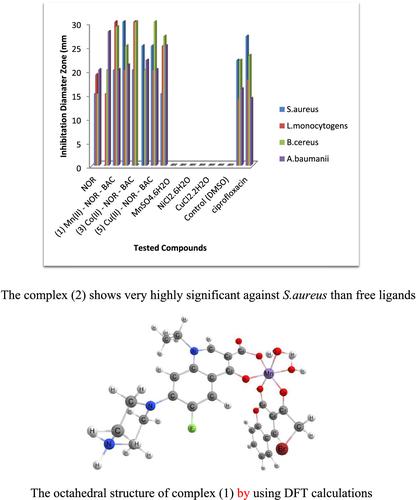
A series of new mixed ligand metal complexes of Mn(II), Fe(II), Co(II), Ni(II), Cu(II), and Zn(II) have been synthesized by the reaction of norfloxacin (NOR) with 3-(bromoacetyl)coumarin (BAC) in 1:1:1 (Mn+:NOR:BAC) molar ratio, which characterized by elemental analysis, spectroscopic measurements (FT-IR, UV–Vis) molar conductance, effective magnetic moments, and thermogravimetric analysis (TG) and (DTG). All the complexes soluble in DMSO and the conductivity measurement indicate that the complexes are electrolyte with ratio 1:1 for Mn(II), Fe(II), and Zn(II) and 1:2 for Co(II), Ni(II), and Cu(II). Electronic and magnetic data elucidated the octahedral structure for all complexes. Assorted thermodynamic factors are calculated, and the results are explicated. Molecular modeling calculations (bond angles, dihedral angles, total energy, heat of formation, and dipole moment) confirm the structural geometry of the complexes and indicate the very good agreement between the computed and experimental geometrical parameters. The calculated data for the hardness (η) and absolute softness (σ) showed that all complexes are soft with respect to ligands. The two ligands and their metal complexes have also been screened for their antibacterial and antifungal activity against some selected species; the data showed that the complexes have remarkable potency as compared with the parent ligands.
中文翻译:

研究分子模型和一些生物金属对诺氟沙星在 3-(溴乙酰)香豆素存在下的效率的影响
通过诺氟沙星 (NOR) 的反应合成了一系列新的 Mn(II)、Fe(II)、Co(II)、Ni(II)、Cu(II) 和 Zn(II) 的混合配体金属配合物与 3-(溴乙酰)香豆素 (BAC) 以 1:1:1 (M n+:NOR:BAC) 摩尔比,其特征在于元素分析、光谱测量(FT-IR、UV-Vis)摩尔电导、有效磁矩以及热重分析 (TG) 和 (DTG)。所有可溶于DMSO的配合物和电导率测量表明,配合物是电解质,Mn(II)、Fe(II)和Zn(II)的比例为1:1,Co(II)、Ni(II)的比例为1:2 ) 和 Cu(II)。电子和磁性数据阐明了所有配合物的八面体结构。计算各种热力学因素,并解释结果。分子建模计算(键角、二面角、总能量、形成热和偶极矩)证实了复合物的结构几何形状,并表明计算的和实验的几何参数之间非常吻合。硬度 (η) 和绝对软度 (σ) 的计算数据表明,所有配合物对于配体都是软的。还筛选了这两种配体及其金属配合物对某些选定物种的抗菌和抗真菌活性;数据表明,与母体配体相比,复合物具有显着的效力。
更新日期:2021-09-14
中文翻译:

研究分子模型和一些生物金属对诺氟沙星在 3-(溴乙酰)香豆素存在下的效率的影响
通过诺氟沙星 (NOR) 的反应合成了一系列新的 Mn(II)、Fe(II)、Co(II)、Ni(II)、Cu(II) 和 Zn(II) 的混合配体金属配合物与 3-(溴乙酰)香豆素 (BAC) 以 1:1:1 (M n+:NOR:BAC) 摩尔比,其特征在于元素分析、光谱测量(FT-IR、UV-Vis)摩尔电导、有效磁矩以及热重分析 (TG) 和 (DTG)。所有可溶于DMSO的配合物和电导率测量表明,配合物是电解质,Mn(II)、Fe(II)和Zn(II)的比例为1:1,Co(II)、Ni(II)的比例为1:2 ) 和 Cu(II)。电子和磁性数据阐明了所有配合物的八面体结构。计算各种热力学因素,并解释结果。分子建模计算(键角、二面角、总能量、形成热和偶极矩)证实了复合物的结构几何形状,并表明计算的和实验的几何参数之间非常吻合。硬度 (η) 和绝对软度 (σ) 的计算数据表明,所有配合物对于配体都是软的。还筛选了这两种配体及其金属配合物对某些选定物种的抗菌和抗真菌活性;数据表明,与母体配体相比,复合物具有显着的效力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号