Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Anion-Specific Water Interactions with Nanochitin: Donnan and Osmotic Pressure Effects as Revealed by Quartz Microgravimetry
Langmuir ( IF 3.7 ) Pub Date : 2021-09-14 , DOI: 10.1021/acs.langmuir.1c01585 Soo-Ah Jin 1 , Saad A Khan 1 , Richard J Spontak 1, 2 , Orlando J Rojas 1, 3, 4
Langmuir ( IF 3.7 ) Pub Date : 2021-09-14 , DOI: 10.1021/acs.langmuir.1c01585 Soo-Ah Jin 1 , Saad A Khan 1 , Richard J Spontak 1, 2 , Orlando J Rojas 1, 3, 4
Affiliation
|
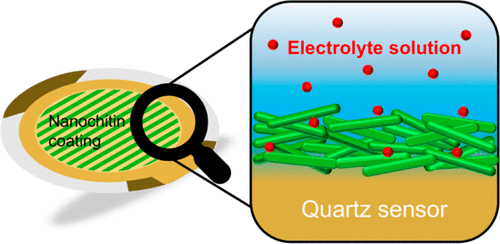
The development of new materials emphasizes greater use of sustainable and eco-friendly resources, including those that take advantage of the unique properties of nanopolysaccharides. Advances in this area, however, necessarily require a thorough understanding of interactions with water. Our contribution to this important topic pertains to the swelling behavior of partially deacetylated nanochitin (NCh), which has been studied here by quartz crystal microgravimetry. Ultrathin films of NCh supported on gold-coated resonators have been equilibrated in aqueous electrolyte solutions (containing NaF, NaCl, NaBr, NaNO3, Na2SO4, Na2SO3, or Na3PO4) at different ionic strengths. As anticipated, NCh displays contrasting swelling/deswelling responses, depending on the ionic affinities and valences of the counterions. The extent of water uptake induced by halide anions, for instance, follows a modified Hofmeister series with F– producing the highest swelling. In marked contrast, Cl– induces film dehydration. We conclude that larger anions promote deswelling such that water losses increase with increasing anion valence. Results such as the ones reported here are critical to ongoing efforts designed to dry chitin nanomaterials and develop bio-based and sustainable materials, including particles, films, coatings, and other nanostructured assemblies, for various devices and applications.
中文翻译:

阴离子特异性水与纳米壳素的相互作用:石英显微重量法揭示的唐南和渗透压效应
新材料的开发强调更多地利用可持续和生态友好的资源,包括那些利用纳米多糖独特特性的资源。然而,该领域的进步必然需要对与水的相互作用有透彻的了解。我们对这一重要课题的贡献与部分脱乙酰纳米壳多糖 (NCh) 的溶胀行为有关,这里已通过石英晶体显微重量法对其进行了研究。负载在镀金谐振器上的 NCh 超薄膜已在电解质水溶液(含 NaF、NaCl、NaBr、NaNO 3、Na 2 SO 4、Na 2 SO 3或 Na 3 PO 4) 在不同的离子强度下。正如预期的那样,NCh 显示出对比鲜明的溶胀/消溶胀反应,这取决于反离子的离子亲和力和价态。的水的吸收引起的卤素阴离子的程度,例如,如下的变形霍夫迈斯特系列有F -产生了最高的肿胀。与此形成鲜明对比的是,Cl -诱导薄膜脱水。我们得出结论,较大的阴离子会促进消溶胀,因此随着阴离子化合价的增加,失水量也会增加。诸如此处报告的结果对于旨在干燥几丁质纳米材料和开发生物基和可持续材料(包括颗粒、薄膜、涂层和其他纳米结构组件)的持续努力至关重要,适用于各种设备和应用。
更新日期:2021-09-28
中文翻译:

阴离子特异性水与纳米壳素的相互作用:石英显微重量法揭示的唐南和渗透压效应
新材料的开发强调更多地利用可持续和生态友好的资源,包括那些利用纳米多糖独特特性的资源。然而,该领域的进步必然需要对与水的相互作用有透彻的了解。我们对这一重要课题的贡献与部分脱乙酰纳米壳多糖 (NCh) 的溶胀行为有关,这里已通过石英晶体显微重量法对其进行了研究。负载在镀金谐振器上的 NCh 超薄膜已在电解质水溶液(含 NaF、NaCl、NaBr、NaNO 3、Na 2 SO 4、Na 2 SO 3或 Na 3 PO 4) 在不同的离子强度下。正如预期的那样,NCh 显示出对比鲜明的溶胀/消溶胀反应,这取决于反离子的离子亲和力和价态。的水的吸收引起的卤素阴离子的程度,例如,如下的变形霍夫迈斯特系列有F -产生了最高的肿胀。与此形成鲜明对比的是,Cl -诱导薄膜脱水。我们得出结论,较大的阴离子会促进消溶胀,因此随着阴离子化合价的增加,失水量也会增加。诸如此处报告的结果对于旨在干燥几丁质纳米材料和开发生物基和可持续材料(包括颗粒、薄膜、涂层和其他纳米结构组件)的持续努力至关重要,适用于各种设备和应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号