Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adsorption and Aggregation Behavior of Mixtures of Quaternary-Ammonium-Salt-Type Amphiphilic Compounds with Fluorinated Counterions and Surfactants
Langmuir ( IF 3.7 ) Pub Date : 2021-09-14 , DOI: 10.1021/acs.langmuir.1c01912 Risa Kawai 1 , Shiho Yada 1 , Tomokazu Yoshimura 1
Langmuir ( IF 3.7 ) Pub Date : 2021-09-14 , DOI: 10.1021/acs.langmuir.1c01912 Risa Kawai 1 , Shiho Yada 1 , Tomokazu Yoshimura 1
Affiliation
|
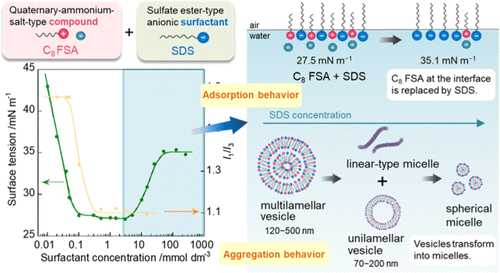
The surface adsorption and aggregation behavior of a mixture of quaternary-ammonium-salt-type amphiphilic monomeric compounds (C4 FSA, C8 FSA, and C4 NTf2) or gemini compounds (C10-2-C4 FSA) and various surfactants (nonionic hexaoxyethylene dodecyl ether (C12EO6), anionic sodium dodecyl sulfate (SDS), cationic dodecyltrimethylammonium bromide (C12TAB), and zwitterionic N-dodecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate (C12Sb)) was investigated. Both types of compounds contained alkyl chains of nonidentical lengths that used bis(fluorosulfonyl)imide (FSA–) or bis(trifluoromethanesulfonyl)imide (NTf2–) as counterions. The mixtures were analyzed for surface tension, viscosity, electrical conductivity, and pyrene fluorescence, in addition to evaluation by cryogenic transmission electron microscopy, small-angle X-ray scattering, and dynamic light scattering. Our results showed that the surface tension depended on the surfactant structure. For the mixture of C8 FSA and SDS, as the SDS concentration increased, the surface tension first decreased and became constant at the critical micelle concentration (CMC). In this concentration range, C8 FSA and SDS were approximately equimolar (2.5 mmol dm–3), the mixture adsorbed efficiently at the air–water interface, and vesicles and linear-type micelles were formed in the solution owing to the decreased electrostatic repulsion between the hydrophilic groups. As the SDS concentration further increased, the surface tension increased and reached another constant value. The C8 FSA at the interface was replaced by SDS and the aggregates transformed into spherical micelles. The surface tension plot of the mixture of the amphiphilic compounds and C12Sb showed a minimum at the CMC. The lowest CMC and surface tension were observed for C10-2-C4 FSA, indicating that the gemini compounds offer excellent adsorption and orientation at the air–water interface. It was revealed that the quaternary-ammonium-salt-type amphiphilic compounds in this study acted as ionic liquids on their own and as surfactants in aqueous solution. Further, they could improve the surface activity of conventional ionic surfactants.
中文翻译:

含氟抗衡离子和表面活性剂的季铵盐型两亲化合物混合物的吸附和聚集行为
季铵盐型两亲单体化合物(C 4 FSA、C 8 FSA 和 C 4 NTf 2)或双子化合物(C 10 -2-C 4 FSA)与各种混合物的表面吸附和聚集行为表面活性剂(非离子型六氧乙烯十二烷基醚(C 12 EO 6),阴离子十二烷基硫酸钠(SDS),阳离子十二烷基三甲基溴(C 12 TAB),和两性离子ñ -dodecyl- ñ,ñ -二甲基-3-铵基-1-丙磺酸盐( ç 12Sb)) 进行了研究。两种类型的化合物都包含长度不同的烷基链,它们使用双(氟磺酰基)酰亚胺(FSA –)或双(三氟甲磺酰基)酰亚胺(NTf 2 –)作为抗衡离子。除了通过低温透射电子显微镜、小角度 X 射线散射和动态光散射进行评估外,还分析了混合物的表面张力、粘度、电导率和芘荧光。我们的结果表明表面张力取决于表面活性剂的结构。对于 C 8 FSA 和 SDS的混合物,随着 SDS 浓度的增加,表面张力首先下降并在临界胶束浓度 (CMC) 下变得恒定。在此浓度范围内,C 8FSA 和 SDS 近似等摩尔(2.5 mmol dm –3),混合物在空气-水界面上有效吸附,由于亲水基团之间的静电排斥力降低,溶液中形成囊泡和线型胶束。随着 SDS 浓度进一步增加,表面张力增加并达到另一个恒定值。界面处的C 8 FSA 被 SDS 取代,聚集体转化为球形胶束。两亲化合物和 C 12 Sb的混合物的表面张力图在 CMC 处显示最小值。对于 C 10 -2-C 4观察到最低的 CMC 和表面张力FSA,表明双子化合物在空气 - 水界面提供出色的吸附和取向。结果表明,本研究中的季铵盐型两亲化合物本身可作为离子液体,在水溶液中可作为表面活性剂。此外,它们可以改善常规离子表面活性剂的表面活性。
更新日期:2021-09-28
中文翻译:

含氟抗衡离子和表面活性剂的季铵盐型两亲化合物混合物的吸附和聚集行为
季铵盐型两亲单体化合物(C 4 FSA、C 8 FSA 和 C 4 NTf 2)或双子化合物(C 10 -2-C 4 FSA)与各种混合物的表面吸附和聚集行为表面活性剂(非离子型六氧乙烯十二烷基醚(C 12 EO 6),阴离子十二烷基硫酸钠(SDS),阳离子十二烷基三甲基溴(C 12 TAB),和两性离子ñ -dodecyl- ñ,ñ -二甲基-3-铵基-1-丙磺酸盐( ç 12Sb)) 进行了研究。两种类型的化合物都包含长度不同的烷基链,它们使用双(氟磺酰基)酰亚胺(FSA –)或双(三氟甲磺酰基)酰亚胺(NTf 2 –)作为抗衡离子。除了通过低温透射电子显微镜、小角度 X 射线散射和动态光散射进行评估外,还分析了混合物的表面张力、粘度、电导率和芘荧光。我们的结果表明表面张力取决于表面活性剂的结构。对于 C 8 FSA 和 SDS的混合物,随着 SDS 浓度的增加,表面张力首先下降并在临界胶束浓度 (CMC) 下变得恒定。在此浓度范围内,C 8FSA 和 SDS 近似等摩尔(2.5 mmol dm –3),混合物在空气-水界面上有效吸附,由于亲水基团之间的静电排斥力降低,溶液中形成囊泡和线型胶束。随着 SDS 浓度进一步增加,表面张力增加并达到另一个恒定值。界面处的C 8 FSA 被 SDS 取代,聚集体转化为球形胶束。两亲化合物和 C 12 Sb的混合物的表面张力图在 CMC 处显示最小值。对于 C 10 -2-C 4观察到最低的 CMC 和表面张力FSA,表明双子化合物在空气 - 水界面提供出色的吸附和取向。结果表明,本研究中的季铵盐型两亲化合物本身可作为离子液体,在水溶液中可作为表面活性剂。此外,它们可以改善常规离子表面活性剂的表面活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号