Journal of CO2 Utilization ( IF 7.2 ) Pub Date : 2021-09-13 , DOI: 10.1016/j.jcou.2021.101707 Hiroyasu Fujitsuka 1 , Takahito Kobayashi 1 , Teruoki Tago 1
|
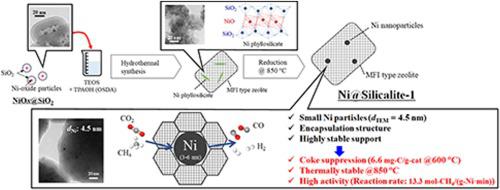
Dry reforming of methane (DRM) is an important reaction for converting CO2 into synthesis gas (H2 + CO). Though Ni is one of the most promising catalysts, the agglomeration of Ni particles and the formation of coke on the catalyst proceed during DRM, resulting in rapid catalyst deactivation. Therefore, the development of Ni-based catalysts that exhibit high thermal stability and suppress coke formation is indispensable. The encapsulation structure of Ni nanoparticles in porous supports is reported to be effective for high DRM activities and the suppression of coke formation.
Herein, we synthesized a Silicalite-1-encapsulated Ni catalyst (Ni@Silicalite-1) for DRM using amorphous silica-encapsulated Ni-oxide nanoparticles (NiOx@SiO2) as the Ni precursor, and the effect of physicochemical properties on DRM activities was investigated using Ni@Silicalite-1, Ni@SiO2 (NiOx@SiO2 after H2 reduction), and Ni/Silicalite-1 (impregnation). For Ni@Silicalite-1, Ni phyllosilicate was formed inside Silicalite-1 during the hydrothermal synthesis, and the encapsulation structure of 4.5-nm-sized Ni particles was realized by reducing Ni phyllosilicate to Ni particles inside the zeolite. Because the small Ni particles were stabilized in the porous supports, Ni@Silicalite-1 and Ni@SiO2 exhibited high and stable activity for DRM at 600 °C with negligible coke formation (6.6 and 8.2 mg-C/g-catalyst for Ni@Silicalite-1 and Ni@SiO2 respectively), whereas a large amount of coke was formed on Ni/Silicalite-1 (532 mg-C/g-catalyst). Ni@Silicalite-1 exhibited high and stable DRM activity throughout 24 h at 600 °C and GHSV = 108000 mL/(g-cat·h). Moreover, because Silicalite1 possessed high thermal stability, the high activity of Ni@Silicalite-1 was maintained throughout the 5 h of DRM at 850 °C, whereas the activity of Ni@SiO2 decreased because of the collapse of the pore structure of SiO2. Ni@Silicalite1 exhibited a higher conversion rate of methane (13.3 mol-CH4/(g-Ni·min) at 850 °C and GHSV = 1080000 mL/(g-cat·h)) than previously reported Ni-based catalysts.
中文翻译:

用无定形二氧化硅包覆镍开发 Silicalite-1-encapsulated Ni 纳米颗粒催化剂用于甲烷干重整:实现焦炭形成抑制和高热稳定性
甲烷干法重整 (DRM) 是将 CO 2转化为合成气 (H 2 + CO)的重要反应。尽管 Ni 是最有前途的催化剂之一,但在 DRM 过程中,Ni 颗粒会发生团聚并在催化剂上形成焦炭,从而导致催化剂快速失活。因此,开发具有高热稳定性和抑制焦炭形成的镍基催化剂是必不可少的。据报道,多孔载体中 Ni 纳米颗粒的封装结构对高 DRM 活性和抑制焦炭形成有效。
在此,我们使用无定形二氧化硅包裹的镍氧化物纳米粒子(NiO x @SiO 2)作为 Ni 前驱体,合成了一种用于 DRM 的 Silicalite-1 包裹的 Ni 催化剂(Ni@Silicalite-1),以及理化性质对 DRM 的影响使用 Ni@Silicalite-1, Ni@SiO 2 (NiO x @SiO 2 after H 2还原)和 Ni/Silicalite-1(浸渍)。对于Ni@Silicalite-1,在水热合成过程中,在Silicalite-1内部形成了Ni层状硅酸盐,通过将Ni层状硅酸盐还原为沸石内部的Ni颗粒,实现了4.5nm大小的Ni颗粒的包封结构。由于小 Ni 颗粒稳定在多孔载体中,Ni@Silicalite-1 和 Ni@SiO 2在 600 °C 下对 DRM 表现出高而稳定的活性,焦炭形成可忽略不计(Ni@Silicalite-1和 8.2 mg-C/g-催化剂) @Silicalite-1 和 Ni@SiO 2分别),而在 Ni/Silicalite-1(532 mg-C/g-催化剂)上形成了大量的焦炭。Ni@Silicalite-1 在 600 °C 和 GHSV = 108000 mL/(g-cat·h) 下在 24 小时内表现出高且稳定的 DRM 活性。此外,由于 Silicalite1 具有高热稳定性,Ni@Silicalite-1 的高活性在 850 °C 的 DRM 5 小时内保持高活性,而 Ni@SiO 2的活性由于 SiO 孔结构的崩溃而降低2 . 与之前报道的镍基催化剂相比,Ni@Silicalite1 表现出更高的甲烷转化率(13.3 mol-CH 4 /(g-Ni·min) 在 850 °C 和 GHSV = 1080000 mL/(g-cat·h))。











































 京公网安备 11010802027423号
京公网安备 11010802027423号