Journal of Allergy and Clinical Immunology ( IF 14.2 ) Pub Date : 2021-09-14 , DOI: 10.1016/j.jaci.2021.07.045 Philippe Gevaert 1 , Rebecca Saenz 2 , Jonathan Corren 3 , Joseph K Han 4 , Joaquim Mullol 5 , Stella E Lee 6 , Randall A Ow 7 , Rui Zhao 2 , Monet Howard 8 , Kit Wong 2 , Lutaf Islam 9 , Monica Ligueros-Saylan 10 , Theodore A Omachi 2 , Claus Bachert 11
|
Background
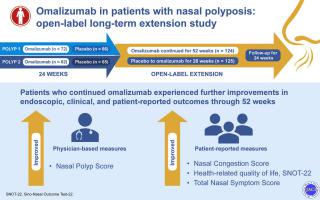
Chronic rhinosinusitis with nasal polyps (CRSwNP) frequently remains uncontrolled despite maximal medical therapy and sinonasal surgery, presenting several unmet needs and challenges. Omalizumab previously demonstrated efficacy in CRSwNP in duplicate phase 3, randomized, placebo-controlled trials (POLYP 1, POLYP 2).
Objective
This open-label extension evaluated the continued efficacy, safety, and durability of response of omalizumab in adults with CRSwNP who completed POLYP 1 or 2.
Methods
After 24 weeks of omalizumab or placebo in POLYP 1 and 2, patients (n = 249) received open-label omalizumab plus background nasal mometasone therapy for 28 weeks and were subsequently followed for 24 weeks after omalizumab discontinuation. Efficacy end points assessed change from baseline for the coprimary end points, Nasal Polyp Score and Nasal Congestion Score, and the secondary end points of Sino-Nasal Outcome Test 22, Total Nasal Symptom Score and its components, and University of Pennsylvania Smell Identification Test scores. Safety objectives included incidence of adverse events and adverse events leading to omalizumab discontinuation.
Results
Patients who continued omalizumab experienced further improvements across coprimary end points and secondary end points through 52 weeks. Patients who switched from placebo to omalizumab experienced favorable responses across end points through week 52 that were similar to POLYP 1 and 2 at week 24. After omalizumab discontinuation, scores gradually worsened over the 24-week follow-up, but remained improved from pretreatment levels for both groups. The safety profile was similar to previous reports.
Conclusions
The efficacy and safety profile from this study supports extended omalizumab treatment up to 1 year for CRSwNP with inadequate response to nasal corticosteroids.
中文翻译:

开放标签扩展研究中奥马珠单抗治疗鼻息肉病的长期疗效和安全性
背景
尽管进行了最大限度的药物治疗和鼻腔鼻窦手术,但伴有鼻息肉的慢性鼻窦炎 (CRSwNP) 经常仍未得到控制,存在一些未满足的需求和挑战。Omalizumab 先前在重复的 3 期、随机、安慰剂对照试验(POLYP 1、POLYP 2)中证明了对 CRSwNP 的疗效。
客观的
该开放标签扩展评估了奥马珠单抗在完成 POLYP 1 或 2 的 CRSwNP 成人中的持续疗效、安全性和反应持久性。
方法
在 POLYP 1 和 2 中使用 omalizumab 或安慰剂 24 周后,患者(n = 249)接受了开放标签的 omalizumab 加背景鼻莫米松治疗 28 周,随后在 omalizumab 停药后随访 24 周。疗效终点评估了联合主要终点、鼻息肉评分和鼻塞评分、鼻腔结果测试 22、总鼻症状评分及其组成部分和宾夕法尼亚大学气味识别测试评分的次要终点的变化. 安全性目标包括不良事件的发生率和导致奥马珠单抗停药的不良事件。
结果
在 52 周内,继续使用 omalizumab 的患者在共同主要终点和次要终点方面经历了进一步的改善。从安慰剂转为 omalizumab 的患者在第 52 周的各个终点都获得了良好的反应,这与第 24 周的 POLYP 1 和 2 相似。在 omalizumab 停药后,评分在 24 周的随访中逐渐恶化,但仍比治疗前水平有所改善对于两组。安全概况与以前的报告相似。
结论
该研究的疗效和安全性支持对鼻用皮质类固醇反应不足的 CRSwNP 延长奥马珠单抗治疗长达 1 年。



























 京公网安备 11010802027423号
京公网安备 11010802027423号