Computational and Structural Biotechnology Journal ( IF 6 ) Pub Date : 2021-09-14 , DOI: 10.1016/j.csbj.2021.09.008 Wanatchaporn Arunmanee 1 , Methawee Duangkaew 1 , Pornchanok Taweecheep 1 , Kanokpol Aphicho 1 , Panuwat Lerdvorasap 1 , Jesada Pitchayakorn 1 , Chayada Intasuk 1 , Runglada Jiraratmetacon 1 , Armini Syamsidi 2 , Pithi Chanvorachote 3, 4 , Chatchai Chaotham 1, 4 , Natapol Pornputtapong 1, 5
|
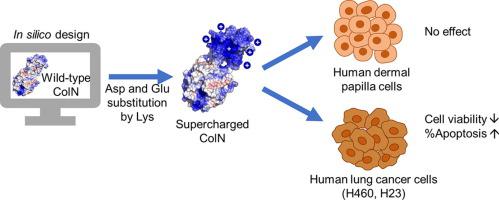
Colicin N (ColN) is a bacteriocin secreted by Escherichia coli (E. coli) to kill other Gram-negative bacteria by forcefully generating ion channels in the inner membrane. In addition to its bactericidal activity, ColN have been reported to selectively induce apoptosis in human lung cancer cells via the suppression of integrin modulated survival pathway. However, ColN showed mild toxicity against human lung cancer cells which could be improved for further applications. The protein resurfacing strategy was chosen to engineer ColN by extensive mutagenesis at solventexposed residues on ColN. The highly accessible Asp and Glu on wildtype ColN (ColNWT) were replaced by Lys to create polycationic ColN (ColN+12). Previous studies have shown that increase of positive charges on proteins leads to the enhancement of mammalian cell penetration as well as increased interaction with negatively charged surface of cancer cells. Those solventexposed residues of ColN were identified by Rosetta and AvNAPSA (Average number of Neighboring Atoms Per Sidechain Atom) approaches. The findings revealed that the structural features and stability of ColN+12 determined by circular dichroism were similar to ColNWT. Furthermore, the toxicity of ColN+12 was cancerselective. Human lung cancer cells, H460 and H23, were sensitive to ColN but human dermal papilla cells were not. ColN+12 also showed more potent toxicity than ColNWT in cancer cells. This confirmed that polycationic resurfacing method has enabled us to improve the anticancer activity of ColN towards human lung cancer cells.
中文翻译:

Colicin N 的受体结合域重铺以增强其对人肺癌细胞的细胞毒作用
Colicin N (ColN) 是大肠杆菌( E.coli )分泌的一种细菌素,通过在内膜中强力产生离子通道来杀死其他革兰氏阴性菌。除了其杀菌活性外,据报道 ColN 还通过抑制整合素调节的存活途径选择性诱导人肺癌细胞凋亡。然而,ColN 对人肺癌细胞表现出轻微的毒性,可以改进以用于进一步的应用。选择蛋白质表面重修策略通过在 ColN 上溶剂暴露的残基处进行广泛诱变来改造 ColN。野生型 ColN (ColN WT )上高度可及的 Asp 和 Glu被 Lys 取代以创建聚阳离子 ColN (ColN +12)。先前的研究表明,蛋白质上正电荷的增加导致哺乳动物细胞渗透的增强以及与癌细胞带负电荷表面的相互作用增加。ColN 的那些溶剂暴露残基通过 Rosetta 和 AvNAPSA(每个侧链原子的平均相邻原子数)方法进行鉴定。研究结果表明,由圆二色性确定的 ColN +12的结构特征和稳定性与 ColN WT相似。此外,ColN +12的毒性是癌症选择性的。人肺癌细胞 H460 和 H23 对 ColN 敏感,但人真皮乳头细胞不敏感。ColN +12也表现出比 ColN WT更强的毒性在癌细胞中。这证实了聚阳离子表面重修方法使我们能够提高 ColN 对人肺癌细胞的抗癌活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号