当前位置:
X-MOL 学术
›
Stem Cells Transl. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase IV postmarketing surveillance study shows continued efficacy and safety of Stempeucel in patients with critical limb ischemia due to Buerger's disease
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2021-09-13 , DOI: 10.1002/sctm.21-0197 Pawan Kumar Gupta 1 , Santanu Dutta 2 , Sanjay Kala 3 , Muralikrishna Nekkanti 4 , Sanjay C Desai 5 , Subhendu S Mahapatra 6 , Anita Dhar 7 , Radhakrishnan Raju 8 , Rajkumar M 9 , Arunanshu Behera 10 , Shivashankar P 1 , N S Raviraja 1 , Pachaiyappan Viswanathan 1 , Mithun Chandrashekar 1 , Charan Thej 1 , Prasanth K V 1 , Jijy Abraham 1 , Hema Boggarapu 1 , K Udaykumar 1
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2021-09-13 , DOI: 10.1002/sctm.21-0197 Pawan Kumar Gupta 1 , Santanu Dutta 2 , Sanjay Kala 3 , Muralikrishna Nekkanti 4 , Sanjay C Desai 5 , Subhendu S Mahapatra 6 , Anita Dhar 7 , Radhakrishnan Raju 8 , Rajkumar M 9 , Arunanshu Behera 10 , Shivashankar P 1 , N S Raviraja 1 , Pachaiyappan Viswanathan 1 , Mithun Chandrashekar 1 , Charan Thej 1 , Prasanth K V 1 , Jijy Abraham 1 , Hema Boggarapu 1 , K Udaykumar 1
Affiliation
|
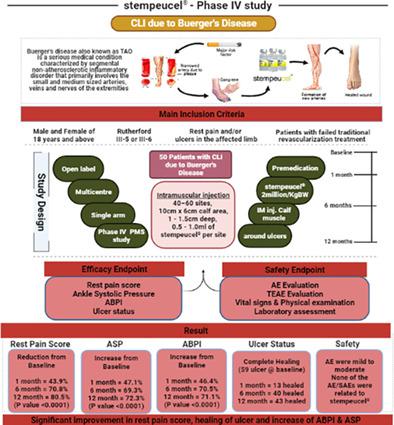
Buerger's disease or thromboangiitis obliterans is a type of obstructive vascular diseases categorized as vasculitis and usually present in 95% of young smoker men. The main pathogenetic mechanism is interplay between immune system and inflammation. Earlier our phase II study has shown that Stempeucel is safe when injected at 2 million cells/kg body weight by virtue of its anti-inflammatory, immunomodulatory, and angiogenetic properties. The present study was conducted to further assess the safety and efficacy of Stempeucel in critical limb ischemia due to Buerger's disease after obtaining approval from Indian FDA based on the data generated in the phase II study. This is an open label, multicenteric phase IV PMS study conducted across India with experienced vascular surgeons. Fifty patients of critical limb ischemia due to Buerger's disease with Rutherford III-5 or III-6 were included in the study and each individual received a dose of 2 million cells/kg body weight of Stempeucel in the calf muscles and around the ulcer. These patients were evaluated over 12 months from drug administration. The present study showed the continued long term efficacy over a period of 12 months follow up in these patients corroborating the result obtained in the previous phase II studies. There was significant improvement in rest pain, ankle systolic pressure, and ankle brachial pressure index with accelerated ulcer healing. In conclusion, the present study shows that the intramuscular administration of Stempeucel continues to be safe, tolerable, and effective alternative treatment in patients with Buerger's disease.
中文翻译:

IV 期上市后监测研究表明,Stempeucel 在因 Buerger 病引起的严重肢体缺血患者中的持续有效性和安全性
伯格氏病或血栓闭塞性脉管炎是一种被归类为血管炎的阻塞性血管疾病,通常存在于 95% 的年轻吸烟男性中。主要的发病机制是免疫系统和炎症之间的相互作用。早些时候,我们的 II 期研究表明,凭借其抗炎、免疫调节和血管生成特性,当以 200 万个细胞/公斤体重注射时,Stempeucel 是安全的。本研究在获得印度 FDA 批准后,根据 II 期研究产生的数据,进一步评估 Stempeucel 在因 Buerger 病引起的严重肢体缺血的安全性和有效性。这是一项开放标签、多中心的 IV 期 PMS 研究,由经验丰富的血管外科医生在印度进行。五十例因 Buerger 引起的严重肢体缺血 研究中包括卢瑟福 III-5 或 III-6 氏病,每个人在小腿肌肉和溃疡周围接受 200 万个细胞/公斤体重的 Stempeucel。这些患者在给药后的 12 个月内接受了评估。本研究表明,这些患者在 12 个月的随访期内持续长期疗效,证实了先前 II 期研究中获得的结果。随着溃疡愈合加快,静息痛、踝关节收缩压和踝肱压力指数均有显着改善。总之,本研究表明,肌肉注射 Stempeucel 对 Buerger 病患者来说仍然是安全、可耐受和有效的替代疗法。
更新日期:2021-09-13
中文翻译:

IV 期上市后监测研究表明,Stempeucel 在因 Buerger 病引起的严重肢体缺血患者中的持续有效性和安全性
伯格氏病或血栓闭塞性脉管炎是一种被归类为血管炎的阻塞性血管疾病,通常存在于 95% 的年轻吸烟男性中。主要的发病机制是免疫系统和炎症之间的相互作用。早些时候,我们的 II 期研究表明,凭借其抗炎、免疫调节和血管生成特性,当以 200 万个细胞/公斤体重注射时,Stempeucel 是安全的。本研究在获得印度 FDA 批准后,根据 II 期研究产生的数据,进一步评估 Stempeucel 在因 Buerger 病引起的严重肢体缺血的安全性和有效性。这是一项开放标签、多中心的 IV 期 PMS 研究,由经验丰富的血管外科医生在印度进行。五十例因 Buerger 引起的严重肢体缺血 研究中包括卢瑟福 III-5 或 III-6 氏病,每个人在小腿肌肉和溃疡周围接受 200 万个细胞/公斤体重的 Stempeucel。这些患者在给药后的 12 个月内接受了评估。本研究表明,这些患者在 12 个月的随访期内持续长期疗效,证实了先前 II 期研究中获得的结果。随着溃疡愈合加快,静息痛、踝关节收缩压和踝肱压力指数均有显着改善。总之,本研究表明,肌肉注射 Stempeucel 对 Buerger 病患者来说仍然是安全、可耐受和有效的替代疗法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号