当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facilitating functionalization of benzene-1,3,5-tricarboxamides by switching amide connectivity
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-09-07 , DOI: 10.1039/d1ob01587g Sandra M C Schoenmakers 1 , Bart W L van den Bersselaar 1 , Shikha Dhiman 1 , Lu Su 1 , Anja R A Palmans 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-09-07 , DOI: 10.1039/d1ob01587g Sandra M C Schoenmakers 1 , Bart W L van den Bersselaar 1 , Shikha Dhiman 1 , Lu Su 1 , Anja R A Palmans 1
Affiliation
|
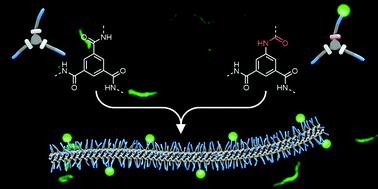
Synthetic water-compatible supramolecular polymers based on benzene-1,3,5-tricarboxamides (BTAs) have attracted a lot of interest in recent years, as they are uniquely suited to generate functional multicomponent biomaterials. Their morphologies and intrinsic dynamic behaviour mimic fibrous structures found in nature. Moreover, their modularity allows control of the density of functionalities presented on the surface of the fibres when using functionalized BTA monomers. However, such moieties generally comprise a functionality on only one of three side chains, resulting in lengthy synthetic protocols and limited yields. In this work, we avert the need for desymmetrization of the core by starting from commercially available 5-aminoisophthalic acid. This approach eliminates the statistical reactions and reduces the number of synthetic steps. It also leads to the inversion of the connectivity of one of the amides to the benzene core. By combining spectroscopy, light scattering and cryogenic transmission electron microscopy, we confirm that the inversed amide BTAs (iBTAs) form intermolecular hydrogen bonds and assemble into supramolecular polymers, like previously used symmetrical BTAs, albeit with a slight decrease in water solubility. Solubility problems were overcome by incorporating iBTAs into conventional BTA-based supramolecular polymers. These two-component mixtures formed supramolecular fibres with a morphology and dynamic behaviour similar to BTA-homopolymers. Finally, iBTAs were decorated with a fluorescent dye to demonstrate the synthesis of functional monomers, and to visualize their co-assembly with BTAs. Our results show that functionality can be introduced into supramolecular polymers with monomers that slightly differ in their core structure while maintaining the structure and dynamics of the fibres.
中文翻译:

通过切换酰胺连接性促进苯-1,3,5-三甲酰胺的功能化
近年来,基于苯-1,3,5-三甲酰胺(BTA)的合成水相容性超分子聚合物引起了广泛关注,因为它们特别适合生产功能性多组分生物材料。它们的形态和内在动态行为模仿自然界中发现的纤维结构。此外,当使用功能化 BTA 单体时,它们的模块化允许控制纤维表面上呈现的功能密度。然而,此类部分通常仅在三个侧链之一上包含功能,导致合成方案冗长且产量有限。在这项工作中,我们从市售的 5-氨基间苯二甲酸开始,避免了核心去对称化的需要。这种方法消除了统计反应并减少了合成步骤的数量。它还导致其中一种酰胺与苯核心的连接性反转。通过结合光谱学、光散射和低温透射电子显微镜,我们证实反酰胺 BTA (iBTA) 形成分子间氢键并组装成超分子聚合物,就像以前使用的对称 BTA 一样,尽管水溶性略有下降。通过将 iBTA 掺入传统的 BTA 基超分子聚合物中,解决了溶解度问题。这些双组分混合物形成的超分子纤维具有与 BTA 均聚物相似的形态和动态行为。最后,iBTA 用荧光染料装饰,以展示功能单体的合成,并可视化它们与 BTA 的共组装。 我们的结果表明,可以将功能引入超分子聚合物中,其单体的核心结构略有不同,同时保持纤维的结构和动力学。
更新日期:2021-09-14
中文翻译:

通过切换酰胺连接性促进苯-1,3,5-三甲酰胺的功能化
近年来,基于苯-1,3,5-三甲酰胺(BTA)的合成水相容性超分子聚合物引起了广泛关注,因为它们特别适合生产功能性多组分生物材料。它们的形态和内在动态行为模仿自然界中发现的纤维结构。此外,当使用功能化 BTA 单体时,它们的模块化允许控制纤维表面上呈现的功能密度。然而,此类部分通常仅在三个侧链之一上包含功能,导致合成方案冗长且产量有限。在这项工作中,我们从市售的 5-氨基间苯二甲酸开始,避免了核心去对称化的需要。这种方法消除了统计反应并减少了合成步骤的数量。它还导致其中一种酰胺与苯核心的连接性反转。通过结合光谱学、光散射和低温透射电子显微镜,我们证实反酰胺 BTA (iBTA) 形成分子间氢键并组装成超分子聚合物,就像以前使用的对称 BTA 一样,尽管水溶性略有下降。通过将 iBTA 掺入传统的 BTA 基超分子聚合物中,解决了溶解度问题。这些双组分混合物形成的超分子纤维具有与 BTA 均聚物相似的形态和动态行为。最后,iBTA 用荧光染料装饰,以展示功能单体的合成,并可视化它们与 BTA 的共组装。 我们的结果表明,可以将功能引入超分子聚合物中,其单体的核心结构略有不同,同时保持纤维的结构和动力学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号