当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Optimization of axial ligands to promote the photoactivation of BODIPY-conjugated platinum(IV) anticancer prodrugs
Dalton Transactions ( IF 3.5 ) Pub Date : 2021-08-30 , DOI: 10.1039/d1dt02362d Houzong Yao 1, 2 , Yuliana F Gunawan 1 , Gongyuan Liu 1, 2 , Man-Kit Tse 1 , Guangyu Zhu 1, 2
Dalton Transactions ( IF 3.5 ) Pub Date : 2021-08-30 , DOI: 10.1039/d1dt02362d Houzong Yao 1, 2 , Yuliana F Gunawan 1 , Gongyuan Liu 1, 2 , Man-Kit Tse 1 , Guangyu Zhu 1, 2
Affiliation
|
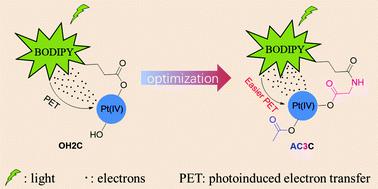
Carboplatin-based platinum(IV) prodrugs containing axial carboxylates are relatively resistant to reduction to release active platinum(II) species and kill cancer cells. To facilitate the activation process, a boron dipyrromethene (BODIPY) ligand has been utilized as a photoabsorber at the axial position to photoactivate carboplatin-based platinum(IV) complexes. However, the influence of the axial ligands on the photoactivation rate of the platinum center and the subsequent biological activity are still unknown. In this study, we report the design and synthesis of a series of carboplatin-based photoactivable platinum(IV) prodrugs containing BODIPY axial ligands with different lengths. The resulting BODIPY-conjugated platinum(IV) prodrugs OH2C–OH8C bearing hydroxido ligands at the opposite axial position are slightly less stable in the dark than the corresponding prodrugs AC2C–AC8C containing acetato ligands. The prodrugs OH3C–OH8C can be photoactivated under irradiation in eight minutes, and the photoactivation rate is further improved in prodrugs AC3C–AC8C where only twenty seconds are needed. Moreover, the prodrug AC3C, in which the linker between the BODIPY photoabsorber and the platinum center has an appropriate length, is photoactivated the quickest among the acetylated prodrugs AC2C–AC8C. The high cellular accumulation may contribute more to the moderate photocytotoxicity of these prodrugs. Our research highlights the way to promote the photoactivation of BODIPY-conjugated platinum(IV) anticancer prodrugs by optimization of axial ligands and may contribute to the future rational design of photoactivable platinum-based complexes.
中文翻译:

优化轴向配体以促进 BODIPY 共轭铂 (IV) 抗癌前药的光活化
含有轴向羧酸盐的基于卡铂的铂 ( IV ) 前药相对难以还原释放活性铂 ( II ) 物质并杀死癌细胞。为了促进活化过程,二吡咯亚甲基硼 (BODIPY) 配体被用作轴向位置的光吸收剂,以光活化卡铂基铂 ( IV ) 配合物。然而,轴向配体对铂中心光活化速率和随后的生物活性的影响仍然未知。在本研究中,我们报道了一系列含有不同长度的BODIPY轴向配体的卡铂基光活化铂( IV )前药的设计和合成。所得的 BODIPY 共轭铂 ( IV ) 前药OH2C–OH8C在相反的轴向位置带有羟基配体,在黑暗中比相应的含有乙酰基配体的前药AC2C–AC8C稳定性稍差。前药OH3C-OH8C在照射下可在8分钟内光活化,前药AC3C-AC8C的光活化速率进一步提高,仅需20秒。此外,前药AC3C ,其中BODIPY光吸收剂和铂中心之间的连接体具有适当的长度,在乙酰化前药AC2C-AC8C中光活化最快。高细胞积累可能更有助于这些前药的中等光细胞毒性。 我们的研究强调了通过优化轴向配体来促进 BODIPY 共轭铂( IV )抗癌前药光活化的方法,并可能有助于未来光活化铂基配合物的合理设计。
更新日期:2021-09-14
中文翻译:

优化轴向配体以促进 BODIPY 共轭铂 (IV) 抗癌前药的光活化
含有轴向羧酸盐的基于卡铂的铂 ( IV ) 前药相对难以还原释放活性铂 ( II ) 物质并杀死癌细胞。为了促进活化过程,二吡咯亚甲基硼 (BODIPY) 配体被用作轴向位置的光吸收剂,以光活化卡铂基铂 ( IV ) 配合物。然而,轴向配体对铂中心光活化速率和随后的生物活性的影响仍然未知。在本研究中,我们报道了一系列含有不同长度的BODIPY轴向配体的卡铂基光活化铂( IV )前药的设计和合成。所得的 BODIPY 共轭铂 ( IV ) 前药OH2C–OH8C在相反的轴向位置带有羟基配体,在黑暗中比相应的含有乙酰基配体的前药AC2C–AC8C稳定性稍差。前药OH3C-OH8C在照射下可在8分钟内光活化,前药AC3C-AC8C的光活化速率进一步提高,仅需20秒。此外,前药AC3C ,其中BODIPY光吸收剂和铂中心之间的连接体具有适当的长度,在乙酰化前药AC2C-AC8C中光活化最快。高细胞积累可能更有助于这些前药的中等光细胞毒性。 我们的研究强调了通过优化轴向配体来促进 BODIPY 共轭铂( IV )抗癌前药光活化的方法,并可能有助于未来光活化铂基配合物的合理设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号