当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Azine-N-oxides as effective controlling groups for Rh-catalysed intermolecular alkyne hydroacylation
Chemical Science ( IF 7.6 ) Pub Date : 2021-09-14 , DOI: 10.1039/d1sc03915f Daniel F Moseley 1 , Jagadeesh Kalepu 1 , Michael C Willis 1
Chemical Science ( IF 7.6 ) Pub Date : 2021-09-14 , DOI: 10.1039/d1sc03915f Daniel F Moseley 1 , Jagadeesh Kalepu 1 , Michael C Willis 1
Affiliation
|
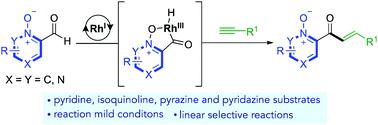
Heterocycle-derived aldehydes are challenging substrates in metal-catalysed hydroacylation chemistry. We show that by using azine N-oxide substituted aldehydes, good reactivity can be achieved, and that they are highly effective substrates for the intermolecular hydroacylation of alkynes. Employing a Rh(I)-catalyst, we achieve a mild and scalable aldehyde C–H activation, that permits the coupling with unactivated terminal alkynes, in good yields and with high regioselectivities (up to >20 : 1 l:b). Both substrates can tolerate a broad variety of functional groups. The reaction can also be applied to diazine aldehydes that contain a free N-lone pair. We demonstrate conversion of the hydroacylation products to the corresponding azine, through a one-pot hydroacylation/deoxygenation sequence. A one-pot hydroacylation/cyclisation, using N-Boc propargylamine, additionally leads to the synthesis of a bidentate pyrrolyl ligand.
中文翻译:

氮嗪-N-氧化物作为 Rh 催化分子间炔烃加氢酰化的有效控制基团
杂环衍生的醛是金属催化加氢酰化化学中具有挑战性的底物。我们表明,通过使用吖嗪N-氧化物取代的醛,可以获得良好的反应性,并且它们是炔烃分子间加氢酰化的高效底物。使用Rh( I )-催化剂,我们实现了温和且可扩展的醛C-H活化,允许与未活化的末端炔烃偶联,具有良好的收率和高区域选择性(高达>20:1l:b)。两种底物都可以耐受多种官能团。该反应也适用于含有游离 N-孤电子对的二嗪醛。我们展示了通过一锅加氢酰化/脱氧序列将加氢酰化产物转化为相应的吖嗪。使用 N-Boc 炔丙胺的一锅加氢酰化/环化另外导致二齿吡咯基配体的合成。
更新日期:2021-09-14
中文翻译:

氮嗪-N-氧化物作为 Rh 催化分子间炔烃加氢酰化的有效控制基团
杂环衍生的醛是金属催化加氢酰化化学中具有挑战性的底物。我们表明,通过使用吖嗪N-氧化物取代的醛,可以获得良好的反应性,并且它们是炔烃分子间加氢酰化的高效底物。使用Rh( I )-催化剂,我们实现了温和且可扩展的醛C-H活化,允许与未活化的末端炔烃偶联,具有良好的收率和高区域选择性(高达>20:1l:b)。两种底物都可以耐受多种官能团。该反应也适用于含有游离 N-孤电子对的二嗪醛。我们展示了通过一锅加氢酰化/脱氧序列将加氢酰化产物转化为相应的吖嗪。使用 N-Boc 炔丙胺的一锅加氢酰化/环化另外导致二齿吡咯基配体的合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号