当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The mechanism of oxidative addition of Pd(0) to Si–H bonds: electronic effects, reaction mechanism, and hydrosilylation
Chemical Science ( IF 7.6 ) Pub Date : 2021-09-14 , DOI: 10.1039/d1sc04419b Michael R Hurst 1 , Lev N Zakharov 1 , Amanda K Cook 1
Chemical Science ( IF 7.6 ) Pub Date : 2021-09-14 , DOI: 10.1039/d1sc04419b Michael R Hurst 1 , Lev N Zakharov 1 , Amanda K Cook 1
Affiliation
|
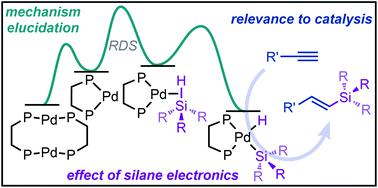
The oxidative addition of Pd to Si–H bonds is a crucial step in a variety of catalytic applications, and many aspects of this reaction are poorly understood. One important yet underexplored aspect is the electronic effect of silane substituents on reactivity. Herein we describe a systematic investigation of the formation of silyl palladium hydride complexes as a function of silane identity, focusing on electronic influence of the silanes. Using [(μ-dcpe)Pd]2 (dcpe = dicyclohexyl(phosphino)ethane) and tertiary silanes, data show that equilibrium strongly favours products formed from electron-deficient silanes, and is fully dynamic with respect to both temperature and product distribution. A notable kinetic isotope effect (KIE) of 1.21 is observed with H/DSiPhMe2 at 233 K, and the reaction is shown to be 0.5th order in [(μ-dcpe)Pd]2 and 1st order in silane. Formed complexes exhibit temperature-dependent intramolecular H/Si ligand exchange on the NMR timescale, allowing determination of the energetic barrier to reversible oxidative addition. Taken together, these results give unique insight into the individual steps of oxidative addition and suggest the initial formation of a σ-complex intermediate to be rate-limiting. The insight gained from these mechanistic studies was applied to hydrosilylation of alkynes, which shows parallel trends in the effect of the silanes' substituents. Importantly, this work highlights the relevance of in-depth mechanistic studies of fundamental steps to catalysis.
中文翻译:

Pd(0)氧化加成到Si-H键的机理:电子效应、反应机理和氢化硅烷化
Pd 与 Si-H 键的氧化加成是各种催化应用中的关键步骤,但对该反应的许多方面知之甚少。一个重要但未充分探索的方面是硅烷取代基对反应性的电子效应。在此,我们描述了作为硅烷特性函数的甲硅烷基氢化钯配合物形成的系统研究,重点是硅烷的电子影响。使用 [(μ-dcpe)Pd] 2 (dcpe = 二环己基(膦基)乙烷) 和叔硅烷,数据表明平衡强烈有利于由缺电子硅烷形成的产品,并且在温度和产品分布方面是完全动态的。使用 H/DSiPhMe 2观察到显着的动力学同位素效应 (KIE) 为 1.21在233 K,且反应被示为0.5个顺序[(μ-DCPE)的Pd] 2和1日顺序硅烷。形成的配合物在 NMR 时间尺度上表现出依赖于温度的分子内 H/Si 配体交换,从而可以确定可逆氧化加成的能量屏障。综上所述,这些结果对氧化加成的各个步骤提供了独特的见解,并表明 σ 复合物中间体的初始形成是限速的。从这些机理研究中获得的见解被应用于炔烃的氢化硅烷化,这显示了硅烷取代基影响的平行趋势。重要的是,这项工作强调了对催化基本步骤进行深入机理研究的相关性。
更新日期:2021-09-14
中文翻译:

Pd(0)氧化加成到Si-H键的机理:电子效应、反应机理和氢化硅烷化
Pd 与 Si-H 键的氧化加成是各种催化应用中的关键步骤,但对该反应的许多方面知之甚少。一个重要但未充分探索的方面是硅烷取代基对反应性的电子效应。在此,我们描述了作为硅烷特性函数的甲硅烷基氢化钯配合物形成的系统研究,重点是硅烷的电子影响。使用 [(μ-dcpe)Pd] 2 (dcpe = 二环己基(膦基)乙烷) 和叔硅烷,数据表明平衡强烈有利于由缺电子硅烷形成的产品,并且在温度和产品分布方面是完全动态的。使用 H/DSiPhMe 2观察到显着的动力学同位素效应 (KIE) 为 1.21在233 K,且反应被示为0.5个顺序[(μ-DCPE)的Pd] 2和1日顺序硅烷。形成的配合物在 NMR 时间尺度上表现出依赖于温度的分子内 H/Si 配体交换,从而可以确定可逆氧化加成的能量屏障。综上所述,这些结果对氧化加成的各个步骤提供了独特的见解,并表明 σ 复合物中间体的初始形成是限速的。从这些机理研究中获得的见解被应用于炔烃的氢化硅烷化,这显示了硅烷取代基影响的平行趋势。重要的是,这项工作强调了对催化基本步骤进行深入机理研究的相关性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号