Journal of Pharmaceutical Analysis ( IF 6.1 ) Pub Date : 2021-09-14 , DOI: 10.1016/j.jpha.2021.09.004 Nilgun Duman 1 , Zahraa ALzaidi 2 , Busra Aynekin 3 , Duygu Taskin 3 , Busra Demirors 3 , Abdulbaki Yildirim 3 , Izem Olcay Sahin 3 , Faik Bilgili 4 , Eda Tahir Turanli 5 , Tommaso Beccari 6 , Matteo Bertelli 7 , Munis Dundar 3
|
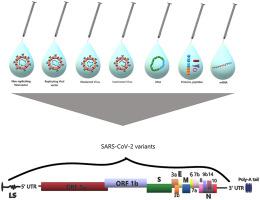
The pandemic caused by the worldwide spread of the coronavirus, which first appeared in 2019, has been named coronavirus disease 19 (COVID-19). More than 4.5 million deaths have been recorded due to the pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), according to the World Health Organization. COVID-19 Dashboard in September 2021. Apart from the wildtype, other variations have been successfully transmitted early in the outbreak although they were not discovered until March 2020. Modifications in the SARS-CoV-2 genetic material, such as mutation and recombination, have the ability to modify the viral life span, along with transitivity, cellular tropism, and symptom severity. Several processes are involved in introducing novel vaccines to the population, including vaccine manufacturing, preclinical studies, Food and Drug Administration permission or certification, processing, and marketing. COVID-19 vaccine candidates have been developed by a number of public and private groups employing a variety of strategies, such as RNA, DNA, protein, and viral vectored vaccines. This comprehensive review, which included the most subsequent evidence on unique features of SARS-CoV-2 and the associated morbidity and mortality, was carried out using a systematic search of recent online databases in order to generate useful knowledge about the COVID-19 updated versions and their consequences on the disease symptoms and vaccine development.
中文翻译:

全球可用的 COVID-19 候选疫苗和疫苗开发平台
由冠状病毒的全球传播引起的大流行于 2019 年首次出现,已被命名为冠状病毒病 19 (COVID-19)。根据世界卫生组织的数据,由于严重急性呼吸系统综合症冠状病毒 2 (SARS-CoV-2) 引起的大流行,已记录了超过 450 万人死亡。2021 年 9 月的 COVID-19 仪表板。除了野生型外,其他变异在爆发早期已成功传播,尽管它们直到 2020 年 3 月才被发现。SARS-CoV-2 遗传物质的修饰,例如突变和重组,已经改变病毒寿命的能力,以及传递性、细胞趋向性和症状严重程度。将新型疫苗引入人群涉及多个过程,包括疫苗制造、临床前研究、食品和药物管理局的许可或认证、加工和营销。COVID-19 候选疫苗已由许多公共和私人团体开发,采用各种策略,例如 RNA、DNA、蛋白质和病毒载体疫苗。这篇综合评价包括关于 SARS-CoV-2 独特特征和相关发病率和死亡率的最新证据,是通过系统搜索最近的在线数据库进行的,以生成有关 COVID-19 更新版本的有用知识及其对疾病症状和疫苗开发的影响。和病毒载体疫苗。这篇综合评价包括关于 SARS-CoV-2 独特特征和相关发病率和死亡率的最新证据,是通过系统搜索最近的在线数据库进行的,以生成有关 COVID-19 更新版本的有用知识及其对疾病症状和疫苗开发的影响。和病毒载体疫苗。这篇综合评价包括关于 SARS-CoV-2 独特特征和相关发病率和死亡率的最新证据,是通过系统搜索最近的在线数据库进行的,以生成有关 COVID-19 更新版本的有用知识及其对疾病症状和疫苗开发的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号