当前位置:
X-MOL 学术
›
Photochem. Photobiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photochemistry of Nitrate Ion: Reduction by Formic Acid under UV Irradiation
Photochemistry and Photobiology ( IF 3.3 ) Pub Date : 2021-09-13 , DOI: 10.1111/php.13518 Na Tong 1, 2 , Zhonglei Xia 3 , Tianzhu Xie 3 , Xu Liu 3 , Jinni Shen 1 , Zizhong Zhang 1 , Xuxu Wang 1
Photochemistry and Photobiology ( IF 3.3 ) Pub Date : 2021-09-13 , DOI: 10.1111/php.13518 Na Tong 1, 2 , Zhonglei Xia 3 , Tianzhu Xie 3 , Xu Liu 3 , Jinni Shen 1 , Zizhong Zhang 1 , Xuxu Wang 1
Affiliation
|
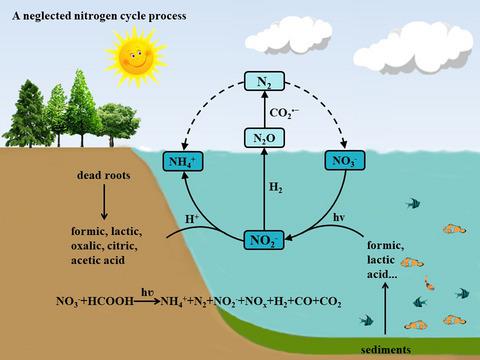
Mutual transformations of various nitrogen compounds and their reaction mechanisms have been a subject of great concern to the chemical, ecological and environmental communities. In the paper, the reactions of ion with small organic acids such as formic acid (HCOOH), acetic acid (CH3COOH) and lactic acid (C3H6O3) under ultraviolet illumination were investigated systematically. It was found that ion is easily reduced into and NOx and then further into N2 and NH3 (in the form of ) in the process. The carboxyl anion radicals and hydrogen formed by photodecomposition of formic acid are responsible for the rapid photoreduction reaction of nitrate. The initial pH and the nitrate concentration considerably affect the product distribution and nitrate conversion. Based on a preliminary simulation study, we speculated that the photoinduced reaction may effectively proceed in oceans, lakes and rivers because of ever-increasing nitrate and organic emissions. This research is helpful to understand nitrogen cycle mechanism and develop water environmental control technologies.
中文翻译:

硝酸根离子的光化学:紫外线照射下的甲酸还原
各种含氮化合物的相互转化及其反应机理一直是化学、生态和环境界非常关注的课题。在论文中,反应系统研究了紫外光照下与甲酸(HCOOH)、乙酸(CH 3 COOH)和乳酸(C 3 H 6 O 3 )等小有机酸的离子。结果发现离子很容易还原成和 NO x,然后进一步转化为 N 2和 NH 3(形式为) 进行中。由甲酸光分解形成的羧基阴离子自由基和氢负责硝酸盐的快速光还原反应。初始 pH 值和硝酸盐浓度显着影响产品分布和硝酸盐转化率。基于初步模拟研究,我们推测由于硝酸盐和有机物排放量不断增加,光致反应可能在海洋、湖泊和河流中有效进行。本研究有助于了解氮循环机制和开发水环境控制技术。
更新日期:2021-09-13
中文翻译:

硝酸根离子的光化学:紫外线照射下的甲酸还原
各种含氮化合物的相互转化及其反应机理一直是化学、生态和环境界非常关注的课题。在论文中,反应系统研究了紫外光照下与甲酸(HCOOH)、乙酸(CH 3 COOH)和乳酸(C 3 H 6 O 3 )等小有机酸的离子。结果发现离子很容易还原成和 NO x,然后进一步转化为 N 2和 NH 3(形式为) 进行中。由甲酸光分解形成的羧基阴离子自由基和氢负责硝酸盐的快速光还原反应。初始 pH 值和硝酸盐浓度显着影响产品分布和硝酸盐转化率。基于初步模拟研究,我们推测由于硝酸盐和有机物排放量不断增加,光致反应可能在海洋、湖泊和河流中有效进行。本研究有助于了解氮循环机制和开发水环境控制技术。



























 京公网安备 11010802027423号
京公网安备 11010802027423号