European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2021-09-13 , DOI: 10.1016/j.ejmech.2021.113846 Margaret K Kurop 1 , Cormac M Huyen 1 , John H Kelly 1 , Brian S J Blagg 1
|
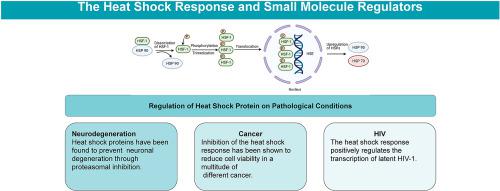
The heat shock response (HSR) is a highly conserved cellular pathway that is responsible for stress relief and the refolding of denatured proteins [1]. When a host cell is exposed to conditions such as heat shock, ischemia, or toxic substances, heat shock factor-1 (HSF-1), a transcription factor, activates the genes that encode for the heat shock proteins (Hsps), which are a family of proteins that work alongside other chaperones to relieve stress and refold proteins that have been denatured (Burdon, 1986) [2]. Along with the refolding of denatured proteins, Hsps facilitate the removal of misfolded proteins by escorting them to degradation pathways, thereby preventing the accumulation of misfolded proteins [3]. Research has indicated that many pathological conditions, such as diabetes, cancer, neuropathy, cardiovascular disease, and aging have a negative impact on HSR function and are commonly associated with misfolded protein aggregation [4,5]. Studies indicate an interplay between mitochondrial homeostasis and HSF-1 levels can impact stress resistance, proteostasis, and malignant cell growth, which further support the role of Hsps in pathological and metabolic functions [6]. On the other hand, Hsp activation by specific small molecules can induce the heat shock response, which can afford neuroprotection and other benefits [7]. This review will focus on the modulation of Hsps and the HSR as therapeutic options to treat these conditions.
中文翻译:

热激反应和小分子调节剂
热休克反应 (HSR) 是一种高度保守的细胞途径,负责缓解应激和变性蛋白质的重折叠 [1]。当宿主细胞暴露于热休克、缺血或有毒物质等条件时,热休克因子-1 (HSF-1)(一种转录因子)会激活编码热休克蛋白 (Hsps) 的基因,这些基因是一个蛋白质家族,与其他伴侣一起发挥作用,缓解压力并重新折叠已变性的蛋白质(Burdon,1986)[2]。随着变性蛋白质的重折叠,热休克蛋白将错误折叠的蛋白质护送至降解途径,从而促进错误折叠蛋白质的去除,从而防止错误折叠蛋白质的积累[3]。研究表明,许多病理状况,如糖尿病、癌症、神经病、心血管疾病和衰老都会对 HSR 功能产生负面影响,并且通常与错误折叠的蛋白质聚集有关 [4,5]。研究表明线粒体稳态和 HSF-1 水平之间的相互作用可以影响应激抵抗、蛋白质稳态和恶性细胞生长,这进一步支持了 Hsps 在病理和代谢功能中的作用 [6]。另一方面,特定小分子激活热休克蛋白可以诱导热休克反应,从而提供神经保护和其他益处[7]。本综述将重点关注 Hsp 和 HSR 的调节作为治疗这些疾病的治疗选择。











































 京公网安备 11010802027423号
京公网安备 11010802027423号