当前位置:
X-MOL 学术
›
J. Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical approaches for the preparation of ubiquitinated proteins via natural linkages
Journal of Peptide Science ( IF 1.8 ) Pub Date : 2021-09-12 , DOI: 10.1002/psc.3367 Yuhui Zhou 1 , Qingsong Xie 1 , Huagui Wang 1 , Hao Sun 1
Journal of Peptide Science ( IF 1.8 ) Pub Date : 2021-09-12 , DOI: 10.1002/psc.3367 Yuhui Zhou 1 , Qingsong Xie 1 , Huagui Wang 1 , Hao Sun 1
Affiliation
|
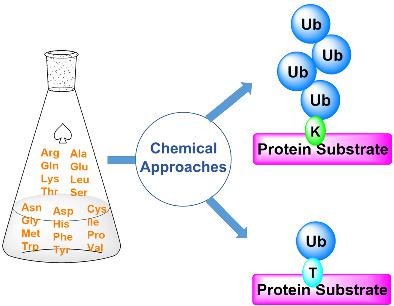
Ubiquitination is an important posttranslation modification (PTM) that regulates a variety of cellular processes, including protein degradation, DNA repair, and viral infections. In this process, the C-terminal carboxyl group of ubiquitin (Ub) or poly-Ub is attached to the ε-amine of lysine (Lys) side chain of an acceptor protein through an isopeptide bond. Studying a molecular mechanism of ubiquitination and deubiquitination is fundamental for unraveling its precise role in health and disease and hence crucial for drug development. Enzymatic approaches for protein ubiquitination possess limited ability to selectivity install Ub or Ub chain on the desired position of an acceptor protein and often lead to heterogeneous mixtures. In the past decades, chemical protein (semi)synthesis has been proved to be an efficient tool to facilitate site-specific protein ubiquitination, which significantly contributes to decode the Ub signal at molecular and structural levels. In this review, we summarize the synthetic strategies developed for protein ubiquitination, and the achievements to generate monoubiquitinated, di-ubiquitinated, and tetraubiquitinated proteins with native isopeptide and ester bonds.
中文翻译:

通过天然连接制备泛素化蛋白质的化学方法
泛素化是一种重要的翻译后修饰 (PTM),可调节多种细胞过程,包括蛋白质降解、DNA 修复和病毒感染。在此过程中,泛素 (Ub) 或聚 Ub 的 C 末端羧基通过异肽键连接到受体蛋白的赖氨酸 (Lys) 侧链的 γ-胺上。研究泛素化和去泛素化的分子机制对于揭示其在健康和疾病中的确切作用至关重要,因此对于药物开发至关重要。用于蛋白质泛素化的酶促方法在受体蛋白的所需位置上选择性安装 Ub 或 Ub 链的能力有限,并且经常导致异质混合物。在过去的几十年里,化学蛋白质(半)合成已被证明是促进位点特异性蛋白质泛素化的有效工具,这对在分子和结构水平上解码 Ub 信号有显着贡献。在这篇综述中,我们总结了为蛋白质泛素化开发的合成策略,以及生成具有天然异肽和酯键的单泛素化、二泛素化和四泛素化蛋白质的成就。
更新日期:2021-09-12
中文翻译:

通过天然连接制备泛素化蛋白质的化学方法
泛素化是一种重要的翻译后修饰 (PTM),可调节多种细胞过程,包括蛋白质降解、DNA 修复和病毒感染。在此过程中,泛素 (Ub) 或聚 Ub 的 C 末端羧基通过异肽键连接到受体蛋白的赖氨酸 (Lys) 侧链的 γ-胺上。研究泛素化和去泛素化的分子机制对于揭示其在健康和疾病中的确切作用至关重要,因此对于药物开发至关重要。用于蛋白质泛素化的酶促方法在受体蛋白的所需位置上选择性安装 Ub 或 Ub 链的能力有限,并且经常导致异质混合物。在过去的几十年里,化学蛋白质(半)合成已被证明是促进位点特异性蛋白质泛素化的有效工具,这对在分子和结构水平上解码 Ub 信号有显着贡献。在这篇综述中,我们总结了为蛋白质泛素化开发的合成策略,以及生成具有天然异肽和酯键的单泛素化、二泛素化和四泛素化蛋白质的成就。











































 京公网安备 11010802027423号
京公网安备 11010802027423号