当前位置:
X-MOL 学术
›
J. Neurosci. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Neuroimmunological complications arising from chemotherapy-induced gut toxicity and opioid exposure in female dark agouti rats
Journal of Neuroscience Research ( IF 2.9 ) Pub Date : 2021-09-12 , DOI: 10.1002/jnr.24959 Juliana Esma Bajic 1, 2 , Gordon Stanley Howarth 1, 3, 4 , Suzanne Mashtoub 1, 4, 5 , Alexandra Louise Whittaker 3 , Larisa Bobrovskaya 6 , Mark Rowland Hutchinson 1, 2
Journal of Neuroscience Research ( IF 2.9 ) Pub Date : 2021-09-12 , DOI: 10.1002/jnr.24959 Juliana Esma Bajic 1, 2 , Gordon Stanley Howarth 1, 3, 4 , Suzanne Mashtoub 1, 4, 5 , Alexandra Louise Whittaker 3 , Larisa Bobrovskaya 6 , Mark Rowland Hutchinson 1, 2
Affiliation
|
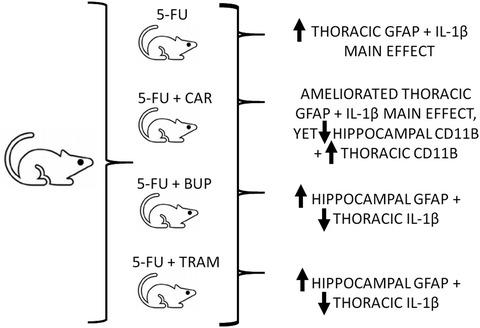
Cancer patients may experience symptom clusters, including chemotherapy-induced (CI) gut toxicity (CIGT) and cognitive impairment. Analgesic selection for pain associated with CIGT is difficult as opioids induce glial reactivity and unwanted side effects. This study quantified central glial reactivity and proinflammatory effects in rats with CIGT using three mechanistically different analgesics. Regional adaptations were indicative of immune-to-brain signaling routes. Utilizing a 5-fluorouracil-induced GT (5IGT) rat model and analgesic intervention (carprofen (CAR), buprenorphine (BUP), and tramadol (TRAM)), spinal and brain neuroimmune modulation was examined via microglial, astrocyte, and proinflammatory (cluster of differentiation molecule 11b; CD11b, glial fibrillary associated protein; GFAP, and interleukin-1 beta; IL1β) reactivity marker expression changes by western blot analysis. 5IGT significantly increased thoracic GFAP (p < 0.05) and IL-1β (p < 0.0001) expression, CAR and BUP ameliorated these effects. BUP and TRAM with 5-FU synergistically increased hippocampal GFAP expression. CAR administered with 5IGT significantly elevated hippocampal and thoracic CD11b expression levels (p < 0.05). The neuroimmune responses observed in this study suggest activation of peripheral-to-central immune signaling pathways. We speculate that the opioid-induced hippocampal changes inferred a humorally mediated mechanism, whereas thoracic neuroimmune modifications indicated activation of an indirect neural route. Although TRAM ameliorated 5IGT-intestinal inflammation, this opioid presents complications relating to bodyweight and regional glial dysregulation (neuroinflammation) and may not be optimal in the management of pain associated with 5IGT. The chemotherapy-induced gut-derived neuroimmune consequences observed suggest a potential mechanistic contribution to central components of the cancer symptom cluster experience, while the opioid-related glial changes have implications for optimal pain management in this setting warranting further investigation.
中文翻译:

雌性暗刺豚鼠化疗引起的肠道毒性和阿片类药物暴露引起的神经免疫并发症
癌症患者可能会出现一系列症状,包括化疗引起的 (CI) 肠道毒性 (CIGT) 和认知障碍。针对 CIGT 相关疼痛的镇痛选择很困难,因为阿片类药物会诱发神经胶质反应和不良副作用。本研究使用三种机制不同的镇痛药量化了 CIGT 大鼠的中枢神经胶质反应性和促炎作用。区域适应表明了免疫至大脑的信号传导途径。利用 5-氟尿嘧啶诱导的 GT (5IGT) 大鼠模型和镇痛干预(卡洛芬 (CAR)、丁丙诺啡 (BUP) 和曲马多 (TRAM)),通过小胶质细胞、星形胶质细胞和促炎细胞(簇)检查脊髓和大脑神经免疫调节。通过蛋白质印迹分析观察分化分子 11b、CD11b、胶质纤维相关蛋白、GFAP 和白细胞介素 1β、IL1β)反应性标记物表达变化。5IGT 显着增加胸部 GFAP ( p < 0.05) 和 IL-1β ( p < 0.0001) 表达,CAR 和 BUP 改善了这些影响。BUP 和 TRAM 与 5-FU 协同增加海马 GFAP 表达。给予 5IGT 的 CAR 显着升高海马和胸部 CD11b 表达水平 ( p < 0.05)。本研究中观察到的神经免疫反应表明外周到中枢免疫信号通路的激活。我们推测阿片类药物引起的海马变化推断出体液介导的机制,而胸部神经免疫改变表明间接神经途径的激活。尽管 TRAM 可以改善 5IGT 肠道炎症,但这种阿片类药物会出现与体重和区域神经胶质失调(神经炎症)相关的并发症,并且在治疗 5IGT 相关疼痛方面可能不是最佳选择。观察到的化疗引起的肠源性神经免疫后果表明对癌症症状群体验的中心组成部分有潜在的机制贡献,而阿片类药物相关的神经胶质变化对这种情况下的最佳疼痛管理有影响,值得进一步研究。
更新日期:2021-09-12
中文翻译:

雌性暗刺豚鼠化疗引起的肠道毒性和阿片类药物暴露引起的神经免疫并发症
癌症患者可能会出现一系列症状,包括化疗引起的 (CI) 肠道毒性 (CIGT) 和认知障碍。针对 CIGT 相关疼痛的镇痛选择很困难,因为阿片类药物会诱发神经胶质反应和不良副作用。本研究使用三种机制不同的镇痛药量化了 CIGT 大鼠的中枢神经胶质反应性和促炎作用。区域适应表明了免疫至大脑的信号传导途径。利用 5-氟尿嘧啶诱导的 GT (5IGT) 大鼠模型和镇痛干预(卡洛芬 (CAR)、丁丙诺啡 (BUP) 和曲马多 (TRAM)),通过小胶质细胞、星形胶质细胞和促炎细胞(簇)检查脊髓和大脑神经免疫调节。通过蛋白质印迹分析观察分化分子 11b、CD11b、胶质纤维相关蛋白、GFAP 和白细胞介素 1β、IL1β)反应性标记物表达变化。5IGT 显着增加胸部 GFAP ( p < 0.05) 和 IL-1β ( p < 0.0001) 表达,CAR 和 BUP 改善了这些影响。BUP 和 TRAM 与 5-FU 协同增加海马 GFAP 表达。给予 5IGT 的 CAR 显着升高海马和胸部 CD11b 表达水平 ( p < 0.05)。本研究中观察到的神经免疫反应表明外周到中枢免疫信号通路的激活。我们推测阿片类药物引起的海马变化推断出体液介导的机制,而胸部神经免疫改变表明间接神经途径的激活。尽管 TRAM 可以改善 5IGT 肠道炎症,但这种阿片类药物会出现与体重和区域神经胶质失调(神经炎症)相关的并发症,并且在治疗 5IGT 相关疼痛方面可能不是最佳选择。观察到的化疗引起的肠源性神经免疫后果表明对癌症症状群体验的中心组成部分有潜在的机制贡献,而阿片类药物相关的神经胶质变化对这种情况下的最佳疼痛管理有影响,值得进一步研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号