Journal of the American College of Cardiology ( IF 21.7 ) Pub Date : 2021-09-13 , DOI: 10.1016/j.jacc.2021.07.022 James P Howard 1 , Frances A Wood 1 , Judith A Finegold 1 , Alexandra N Nowbar 1 , David M Thompson 1 , Ahran D Arnold 1 , Christopher A Rajkumar 1 , Susan Connolly 1 , Jaimini Cegla 2 , Chris Stride 3 , Peter Sever 1 , Christine Norton 4 , Simon A M Thom 1 , Matthew J Shun-Shin 1 , Darrel P Francis 1
|
Background
Most people who begin statins abandon them, most commonly because of side effects.
Objectives
The purpose of this study was to assess daily symptom scores on statin, placebo, and no treatment in participants who had abandoned statins.
Methods
Participants received 12 1-month medication bottles, 4 containing atorvastatin 20 mg, 4 placebo, and 4 empty. We measured daily symptom intensity for each using an app (scale 1-100). We also measured the “nocebo” ratio: the ratio of symptoms induced by taking statin that was also induced by taking placebo.
Results
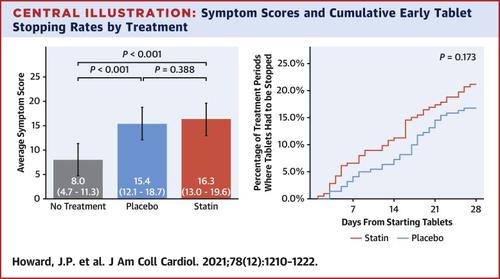
A total of 60 participants were randomized and 49 completed the 12-month protocol. Mean symptom score was 8.0 (95% CI: 4.7-11.3) in no-tablet months. It was higher in statin months (16.3; 95% CI: 13.0-19.6; P < 0.001), but also in placebo months (15.4; 95% CI: 12.1-18.7; P < 0.001), with no difference between the 2 (P = 0.388). The corresponding nocebo ratio was 0.90. In the individual-patient daily data, neither symptom intensity on starting (OR: 1.02; 95% CI: 0.98-1.06; P = 0.28) nor extent of symptom relief on stopping (OR: 1.01; 95% CI: 0.98-1.05; P = 0.48) distinguished between statin and placebo. Stopping was no more frequent for statin than placebo (P = 0.173), and subsequent symptom relief was similar between statin and placebo. At 6 months after the trial, 30 of 60 (50%) participants were back taking statins.
Conclusions
The majority of symptoms caused by statin tablets were nocebo. Clinicians should not interpret symptom intensity or timing of symptom onset or offset (on starting or stopping statin tablets) as indicating pharmacological causation, because the pattern is identical for placebo. (Self-Assessment Method for Statin Side-effects Or Nocebo [SAMSON]; NCT02668016)
中文翻译:

他汀类药物、安慰剂和不治疗交叉试验中的副作用模式
背景
大多数开始服用他汀类药物的人都会放弃,最常见的原因是副作用。
目标
本研究的目的是评估放弃他汀类药物的参与者对他汀类药物、安慰剂和不治疗的每日症状评分。
方法
参与者收到 12 个 1 个月的药瓶,其中 4 个含有阿托伐他汀 20 毫克,4 个为安慰剂,4 个为空瓶。我们使用应用程序测量了每个人的每日症状强度(等级 1-100)。我们还测量了“反安慰剂”比率:服用他汀类药物引起的症状与服用安慰剂引起的症状的比率。
结果
共有 60 名参与者被随机分配,其中 49 名完成了为期 12 个月的方案。在未服用药片的月份中,平均症状评分为 8.0(95% CI:4.7-11.3)。在他汀类药物治疗月份中该值较高(16.3;95% CI:13.0-19.6;P < 0.001),但在安慰剂月份中也较高(15.4;95% CI:12.1-18.7;P < 0.001),两者之间没有差异( P = 0.388)。相应的反安慰剂比率为0.90。在个体患者每日数据中,既没有开始时的症状强度(OR:1.02;95% CI:0.98-1.06;P = 0.28),也没有停止时症状的缓解程度(OR:1.01;95% CI:0.98-1.05;P = 0.28)。P = 0.48)区分他汀类药物和安慰剂。他汀类药物的停药并不比安慰剂更频繁(P = 0.173),并且他汀类药物和安慰剂随后的症状缓解相似。试验后 6 个月,60 名参与者中有 30 名 (50%) 重新服用他汀类药物。
结论
他汀类药物引起的大多数症状都是反安慰剂。临床医生不应将症状强度或症状发作或消失的时间(在开始或停止他汀类药物时)解释为药理学因果关系,因为该模式与安慰剂相同。(他汀类药物副作用或反安慰剂的自我评估方法 [SAMSON];NCT02668016)











































 京公网安备 11010802027423号
京公网安备 11010802027423号