Cell Reports Physical Science ( IF 7.9 ) Pub Date : 2021-09-13 , DOI: 10.1016/j.xcrp.2021.100576 Yanxiong Pan 1 , Xiaoliang Wang 2 , Hui Li 1 , Junyu Ren 3 , Yin Zhang 3 , Drew Jordahl 1 , Isabelle Schuster 1 , Jasmin Farmakes 1 , Heedeok Hong 4 , Zhongyu Yang 1 , Shengqian Ma 3
|
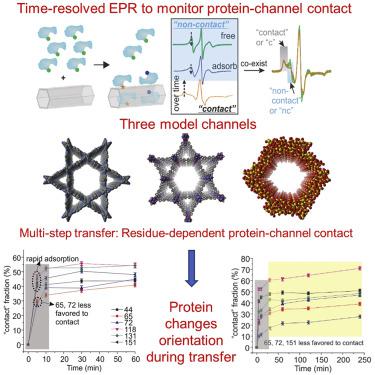
Protein transfer into nanoscale compartments is critical for many cellular/life processes, yet there are few reports on how compartment properties impact the protein orientation during a transfer. Such a knowledge gap limits a deeper understanding of the protein transfer mechanism, which could be bridged using nanoporous materials. Here, we use a mesoporous silica, a covalent organic framework, and a metal-organic framework with charged, hydrophobic, and neutral surfaces, respectively, to elucidate the impact of channel properties on the transfer of a model protein, lysozyme. Using site-directed spin labeling and time-resolved electron paramagnetic resonance spectroscopy, we reveal that the transfer can be a multi-step process depending on channel properties and depict the relative orientation changes of lysozyme upon transfer into each channel. To the best of our knowledge, this is the first structural insight into protein orientation upon transfer into different compartments, meaningful for the rational design of synthetic materials to host enzymes or mimic the cellular compartments.
中文翻译:

蛋白质转移到纳米级通道的原位监测
蛋白质转移到纳米级隔室对于许多细胞/生命过程至关重要,但很少有关于隔室特性如何在转移过程中影响蛋白质方向的报道。这种知识差距限制了对蛋白质转移机制的更深入理解,而这可以使用纳米多孔材料来弥补。在这里,我们分别使用介孔二氧化硅、共价有机框架和具有带电、疏水和中性表面的金属有机框架来阐明通道特性对模型蛋白溶菌酶转移的影响。使用定点自旋标记和时间分辨电子顺磁共振波谱,我们发现转移可以是一个多步骤的过程,具体取决于通道特性,并描述了转移到每个通道后溶菌酶的相对方向变化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号