当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, molecular docking and ADME studies of novel indole-thiazolidinedione derivatives and their antineoplastic activity as CDK6 inhibitors
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2021-09-13 , DOI: 10.1039/d1nj02808a Zeynep Ates-Alagoz 1 , Mehmet Murat Kisla 1 , Fikriye Zengin Karadayi 1 , Sercan Baran 1 , Tuğba Somay Doğan 2 , Pelin Mutlu 2
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2021-09-13 , DOI: 10.1039/d1nj02808a Zeynep Ates-Alagoz 1 , Mehmet Murat Kisla 1 , Fikriye Zengin Karadayi 1 , Sercan Baran 1 , Tuğba Somay Doğan 2 , Pelin Mutlu 2
Affiliation
|
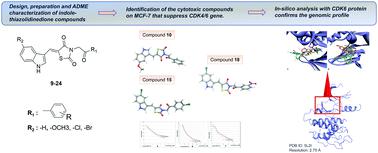
Several 5-((5-substituted-1H-indole-3-yl)methylene)-3-(2-oxo-2-(3/4-substituted-phenylethyl)-thiazolidine-2,4-dione derivatives (9–24) were designed and synthesized as CDK6 inhibitors, and their anticancer activity was probed on the MCF-7 breast cancer cell line and their effects on gene expression profiles were elucidated. According to biological activity assays, compounds 10, 15, and 18 were found to possess favorable cytotoxicity on this cell line. For a better understanding of their activity rationale, genomic studies were conducted. Changes in gene expression levels occurring in MCF-7 cells were studied on 48 genes selected among genes associated with the estrogen receptor, tumor suppressor and oncogenes, microtubule formation, apoptosis, the cell cycle, drug resistance and inflammation. It was determined that there are significant differences in gene expression levels in 21 of these genes. Comparing to other genes, these compounds inhibited gene expression of CDK6 much more. For a more thorough evaluation of their mechanism of action involving this pathway, docking analysis was performed with a corresponding enzyme that is synthesized by the CDK6 gene. By doing so, the binding profiles of these derivatives were compared to the reference study. In the end, the impact of the indole-thiazolidinediones on CDK6 and their mechanisms of action were elucidated. Compounds 15 and 18 possess higher affinity with better binding interactions relative to that of compound 10. These two compounds were highlighted as possible candidates for upcoming design studies of CDK6 inhibitors. Moreover, the druglikeness of the indole-thiazolidinediones was calculated and compared to commercial anticancer drugs.
中文翻译:

新型吲哚-噻唑烷二酮衍生物及其作为 CDK6 抑制剂的抗肿瘤活性的设计、合成、分子对接和 ADME 研究
几种 5-((5-取代-1 H-吲哚-3-基)亚甲基)-3-(2-氧代-2-(3/4-取代-苯乙基)-噻唑烷-2,4-二酮衍生物 ( 9 -24),设计并作为CDK6抑制剂合成的,并且它们的抗癌活性物探测上MCF-7乳腺癌细胞系和分别鉴定其基因表达谱的效果。根据生物学活性测定法中,化合物10,15,和18发现对这种细胞系具有良好的细胞毒性。为了更好地了解它们的活动原理,进行了基因组研究。在与雌激素受体、肿瘤抑制基因和癌基因、微管形成、细胞凋亡、细胞周期、耐药性和炎症相关的基因中选择了 48 个基因,研究了 MCF-7 细胞中基因表达水平的变化。已确定这些基因中的 21 个基因表达水平存在显着差异。与其他基因相比,这些化合物更能抑制 CDK6 的基因表达。为了更彻底地评估它们涉及该途径的作用机制,使用由 CDK6 基因合成的相应酶进行对接分析。通过这样做,将这些衍生物的结合谱与参考研究进行了比较。最后,阐明了吲哚-噻唑烷二酮对 CDK6 的影响及其作用机制。化合物与化合物10相比,图15和18具有更高的亲和力和更好的结合相互作用。这两种化合物被强调为即将进行的 CDK6 抑制剂设计研究的可能候选者。此外,计算了吲哚-噻唑烷二酮的药物相似性,并与商业抗癌药物进行了比较。
更新日期:2021-09-13
中文翻译:

新型吲哚-噻唑烷二酮衍生物及其作为 CDK6 抑制剂的抗肿瘤活性的设计、合成、分子对接和 ADME 研究
几种 5-((5-取代-1 H-吲哚-3-基)亚甲基)-3-(2-氧代-2-(3/4-取代-苯乙基)-噻唑烷-2,4-二酮衍生物 ( 9 -24),设计并作为CDK6抑制剂合成的,并且它们的抗癌活性物探测上MCF-7乳腺癌细胞系和分别鉴定其基因表达谱的效果。根据生物学活性测定法中,化合物10,15,和18发现对这种细胞系具有良好的细胞毒性。为了更好地了解它们的活动原理,进行了基因组研究。在与雌激素受体、肿瘤抑制基因和癌基因、微管形成、细胞凋亡、细胞周期、耐药性和炎症相关的基因中选择了 48 个基因,研究了 MCF-7 细胞中基因表达水平的变化。已确定这些基因中的 21 个基因表达水平存在显着差异。与其他基因相比,这些化合物更能抑制 CDK6 的基因表达。为了更彻底地评估它们涉及该途径的作用机制,使用由 CDK6 基因合成的相应酶进行对接分析。通过这样做,将这些衍生物的结合谱与参考研究进行了比较。最后,阐明了吲哚-噻唑烷二酮对 CDK6 的影响及其作用机制。化合物与化合物10相比,图15和18具有更高的亲和力和更好的结合相互作用。这两种化合物被强调为即将进行的 CDK6 抑制剂设计研究的可能候选者。此外,计算了吲哚-噻唑烷二酮的药物相似性,并与商业抗癌药物进行了比较。











































 京公网安备 11010802027423号
京公网安备 11010802027423号