Inorganic Chemistry Communications ( IF 4.4 ) Pub Date : 2021-09-13 , DOI: 10.1016/j.inoche.2021.108914 Shirin Shabani 1 , Mohammad Dinari 2
|
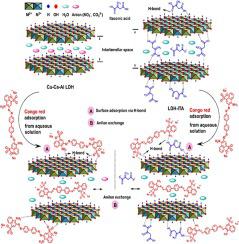
Here, for the first time, Cu-Ca-Al-based layered double hydroxide (LDH) was prepared using a facile co-precipitation method. Itaconic acid (ITA) was used as a biodegradable and green organic molecule to modify the LDH and then physicochemical properties of the ITA-modified LDH (LDH-ITA) were studied using FTIR, FE-SEM, TEM, XRD, and TGA techniques. Then the synthesized LDH and LDH-ITA were applied as novel adsorbents for the removal of Congo red as a model of an anionic dye from aqueous media. Several parameters such as the solution pH, contact time, adsorbent amount, and initial concentration of the dye on the adsorption process were monitored and kinetic and isotherm studies were conducted to gain a more in-depth insight into the adsorption mechanism in the process. The results demonstrated that the adsorption of Congo red onto both the LDH and LDH-ITA adsorbents followed the pseudo-second-order kinetic model. Also, isotherm investigations revealed that the Freundlich model provided the best fit with the equilibrium data of both adsorbents. Maximum adsorption capacities of 81 and 84 mg g−1 for Cong red were obtained using Cu-Ca-Al-LDH and LDH-ITA adsorbents, respectively, surpassing those values obtained for most adsorbents reported in the previous studies.
中文翻译:

衣康酸改性的 Cu-Ca-Al 层状双氢氧化物作为吸附剂去除阴离子染料:动力学和等温线研究
在这里,首次使用简便的共沉淀方法制备了基于 Cu-Ca-Al 的层状双氢氧化物 (LDH)。衣康酸 (ITA) 作为可生物降解的绿色有机分子对 LDH 进行改性,然后使用 FTIR、FE-SEM、TEM、XRD 和 TGA 技术研究了 ITA 改性的 LDH (LDH-ITA) 的理化性质。然后合成的 LDH 和 LDH-ITA 被用作新型吸附剂,用于从水性介质中去除作为阴离子染料模型的刚果红。监测溶液pH、接触时间、吸附剂量和吸附过程中染料的初始浓度等几个参数,并进行动力学和等温线研究,以更深入地了解吸附过程中的吸附机制。结果表明刚果红在LDH和LDH-ITA吸附剂上的吸附遵循准二级动力学模型。此外,等温线研究表明,Freundlich 模型提供了两种吸附剂平衡数据的最佳拟合。最大吸附容量为 81 和 84 mg g分别使用 Cu-Ca-Al-LDH 和 LDH-ITA 吸附剂获得丛红的-1,超过了先前研究中报道的大多数吸附剂获得的值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号