当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Atomic heterointerface engineering overcomes the activity limitation of electrocatalysts and promises highly-efficient alkaline water splitting
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2021-09-02 , DOI: 10.1039/d1ee02105b Qiucheng Xu 1 , Jiahao Zhang 1 , Haoxuan Zhang 1 , Liyue Zhang 1 , Ling Chen 2 , Yanjie Hu 1 , Hao Jiang 1, 2 , Chunzhong Li 1, 2
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2021-09-02 , DOI: 10.1039/d1ee02105b Qiucheng Xu 1 , Jiahao Zhang 1 , Haoxuan Zhang 1 , Liyue Zhang 1 , Ling Chen 2 , Yanjie Hu 1 , Hao Jiang 1, 2 , Chunzhong Li 1, 2
Affiliation
|
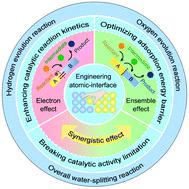
Alkaline water splitting, especially anion-exchange-membrane based water electrolysis, is an attractive way for low-cost and scalable H2 production. Green electricity-driven alkaline water electrolysis requires that highly-efficient electrocatalysts be developed to further decrease the barriers of two half reactions – the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER). Various strategies have been exploited to date, among which atomic heterointerface engineering is the most promising strategy to overcome the intrinsic activity limitation of electrocatalysts. In this review, we thoroughly summarize the recent progress of atomic heterointerface engineering to improve the activity of electrocatalysts. The origin and rationale of the sluggish kinetics of the alkaline HER and OER are first introduced. Subsequently, the synergistic effects (ensemble effect and electron effect) of atomic heterointerface engineering to overcome the activity limitation are elaborated, in which the ensemble effect is helpful in optimizing the reaction pathways with enhanced reaction kinetics by creating a favorable heterointerface and the electron effect can balance the adsorption energies of reaction intermediates by coupling their electronic configurations. And then the rational design of the targeted electrocatalysts is concluded based on the heterointerface constituents and characteristics. At the end, some outlooks about the future development direction for optimizing and maximizing the interfacial active sites in the electrocatalysts are proposed.
中文翻译:

原子异质界面工程克服了电催化剂的活性限制并有望实现高效的碱性水分解
碱性水分解,特别是基于阴离子交换膜的水电解,是低成本和可扩展的 H 2的有吸引力的方式生产。绿色电力驱动的碱性水电解需要开发高效的电催化剂,以进一步降低析氢反应(HER)和析氧反应(OER)这两个半反应的势垒。迄今为止,已经开发了各种策略,其中原子异质界面工程是克服电催化剂内在活性限制的最有前途的策略。在这篇综述中,我们彻底总结了原子异质界面工程在提高电催化剂活性方面的最新进展。首先介绍了碱性 HER 和 OER 缓慢动力学的起源和基本原理。随后,阐述了原子异质界面工程克服活性限制的协同效应(集合效应和电子效应),其中集成效应有助于通过创建有利的异质界面来优化反应动力学并增强反应动力学,而电子效应可以通过耦合反应中间体的电子构型来平衡反应中间体的吸附能。然后根据异质界面的组成和特性得出目标电催化剂的合理设计。最后,对优化和最大化电催化剂界面活性位点的未来发展方向提出了一些展望。然后根据异质界面的组成和特性得出目标电催化剂的合理设计。最后,对优化和最大化电催化剂界面活性位点的未来发展方向提出了一些展望。然后根据异质界面的组成和特性得出目标电催化剂的合理设计。最后,对优化和最大化电催化剂界面活性位点的未来发展方向提出了一些展望。
更新日期:2021-09-13
中文翻译:

原子异质界面工程克服了电催化剂的活性限制并有望实现高效的碱性水分解
碱性水分解,特别是基于阴离子交换膜的水电解,是低成本和可扩展的 H 2的有吸引力的方式生产。绿色电力驱动的碱性水电解需要开发高效的电催化剂,以进一步降低析氢反应(HER)和析氧反应(OER)这两个半反应的势垒。迄今为止,已经开发了各种策略,其中原子异质界面工程是克服电催化剂内在活性限制的最有前途的策略。在这篇综述中,我们彻底总结了原子异质界面工程在提高电催化剂活性方面的最新进展。首先介绍了碱性 HER 和 OER 缓慢动力学的起源和基本原理。随后,阐述了原子异质界面工程克服活性限制的协同效应(集合效应和电子效应),其中集成效应有助于通过创建有利的异质界面来优化反应动力学并增强反应动力学,而电子效应可以通过耦合反应中间体的电子构型来平衡反应中间体的吸附能。然后根据异质界面的组成和特性得出目标电催化剂的合理设计。最后,对优化和最大化电催化剂界面活性位点的未来发展方向提出了一些展望。然后根据异质界面的组成和特性得出目标电催化剂的合理设计。最后,对优化和最大化电催化剂界面活性位点的未来发展方向提出了一些展望。然后根据异质界面的组成和特性得出目标电催化剂的合理设计。最后,对优化和最大化电催化剂界面活性位点的未来发展方向提出了一些展望。











































 京公网安备 11010802027423号
京公网安备 11010802027423号