当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure-guided evolution of a ketoreductase for efficient and stereoselective bioreduction of bulky α-amino β-keto esters
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2021-09-09 , DOI: 10.1039/d1cy01032h Jiawei Tang 1 , Liuqing Chen 2 , Luwen Zhang 3 , Guowei Ni 3 , Jun Yu 3 , Hongyi Wang 3 , Fuli Zhang 3 , Shuguang Yuan 2 , Meiqing Feng 1 , Shaoxin Chen 3
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2021-09-09 , DOI: 10.1039/d1cy01032h Jiawei Tang 1 , Liuqing Chen 2 , Luwen Zhang 3 , Guowei Ni 3 , Jun Yu 3 , Hongyi Wang 3 , Fuli Zhang 3 , Shuguang Yuan 2 , Meiqing Feng 1 , Shaoxin Chen 3
Affiliation
|
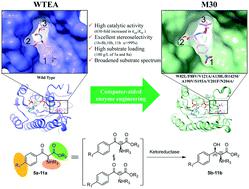
Ketoreductases have shown considerable potential as biocatalysts in the asymmetric synthesis of chiral alcohols. However, compared to the widely studied ketoreductases for chiral alcohols, limited knowledge is available about ketoreductases for efficient dynamic reductive kinetic resolution (DYRKR) of bulky α-amino β-keto esters. In this study, structure-guided rational direct evolution was applied to a ketoreductase (WTEA) from Exiguobacterium sp. F42 for the asymmetric reduction of bulky α-amino β-keto esters. A number of mutants were then obtained with remarkably improved activity toward various bulky α-amino β-keto esters with excellent stereoselectivity by performing structure-guided rational design. In particular, mutant M30 (W82L/F88V/V121A/A138L/R142M/A190V/S193A/Y201F/N204A) exhibited excellent stereoselectivity (>99% dr, >99% de) and high conversion (>99%) for six α-amino β-keto esters. Furthermore, novel and practical chemoenzymatic routes were developed for the synthesis of chloramphenicol and florfenicol, which featured the application of enzymatic DYRKR to establish the two stereocenters of amino alcohols 4-NO2-substituted (2S, 3R)-5b and 4-SO2Me-substituted (2S, 3R)-8b with > 99% dr, > 99% de and >99% conversion from 100 g L−1 α-amino β-keto esters 5a and 8a (highest substrate loading reported), respectively. Crystal structure and molecular dynamics studies revealed the potential molecular basis for activity improvement and the stereoselectivity control mechanism at the atomic level. These results provide important insights into the evolution of ketoreductases for the asymmetric synthesis of chiral vicinal amino alcohols and establish a solid foundation for further large-scale industrial applications in the future.
中文翻译:

酮还原酶的结构引导进化,用于有效和立体选择性生物还原庞大的 α-氨基 β-酮酯
酮还原酶在手性醇的不对称合成中作为生物催化剂显示出相当大的潜力。然而,与广泛研究的手性醇酮还原酶相比,关于酮还原酶用于大体积 α-氨基 β-酮酯的有效动态还原动力学拆分 (DYRKR) 的知识有限。在这项研究中,以结构为导向合理直接演化应用于从酮还原酶(WTEA)微小杆菌属sp. F42 用于不对称还原庞大的 α-氨基 β-酮酯。然后通过进行结构引导的合理设计,获得了许多突变体,它们对各种体积大的 α-氨基 β-酮酯的活性显着提高,具有优异的立体选择性。特别是,突变体 M30 (W82L/F88V/V121A/A138L/R142M/A190V/S193A/Y201F/N204A) 对六种α-氨基β-酮酯。此外,开发了新型实用的化学酶法合成氯霉素和氟苯尼考,其特点是应用酶法DYRKR建立氨基醇4-NO 2 -取代(2 S , 3 R )- 5b和4-的两个立体中心。SO 2Me-取代的 (2 S , 3 R )- 8b从 100 g L -1 α-氨基 β-酮酯5a和8a转化为 > 99% dr、> 99% de 和 >99% (报告的最高底物负载),分别。晶体结构和分子动力学研究揭示了在原子水平上提高活性的潜在分子基础和立体选择性控制机制。这些结果为用于手性邻氨基醇不对称合成的酮还原酶的进化提供了重要的见解,并为未来进一步的大规模工业应用奠定了坚实的基础。
更新日期:2021-09-13
中文翻译:

酮还原酶的结构引导进化,用于有效和立体选择性生物还原庞大的 α-氨基 β-酮酯
酮还原酶在手性醇的不对称合成中作为生物催化剂显示出相当大的潜力。然而,与广泛研究的手性醇酮还原酶相比,关于酮还原酶用于大体积 α-氨基 β-酮酯的有效动态还原动力学拆分 (DYRKR) 的知识有限。在这项研究中,以结构为导向合理直接演化应用于从酮还原酶(WTEA)微小杆菌属sp. F42 用于不对称还原庞大的 α-氨基 β-酮酯。然后通过进行结构引导的合理设计,获得了许多突变体,它们对各种体积大的 α-氨基 β-酮酯的活性显着提高,具有优异的立体选择性。特别是,突变体 M30 (W82L/F88V/V121A/A138L/R142M/A190V/S193A/Y201F/N204A) 对六种α-氨基β-酮酯。此外,开发了新型实用的化学酶法合成氯霉素和氟苯尼考,其特点是应用酶法DYRKR建立氨基醇4-NO 2 -取代(2 S , 3 R )- 5b和4-的两个立体中心。SO 2Me-取代的 (2 S , 3 R )- 8b从 100 g L -1 α-氨基 β-酮酯5a和8a转化为 > 99% dr、> 99% de 和 >99% (报告的最高底物负载),分别。晶体结构和分子动力学研究揭示了在原子水平上提高活性的潜在分子基础和立体选择性控制机制。这些结果为用于手性邻氨基醇不对称合成的酮还原酶的进化提供了重要的见解,并为未来进一步的大规模工业应用奠定了坚实的基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号