Structure ( IF 4.4 ) Pub Date : 2021-09-13 , DOI: 10.1016/j.str.2021.07.008 Yasaman Karami 1 , Aracelys López-Castilla 2 , Andrea Ori 3 , Jenny-Lee Thomassin 3 , Benjamin Bardiaux 1 , Therese Malliavin 1 , Nadia Izadi-Pruneyre 2 , Olivera Francetic 3 , Michael Nilges 1
|
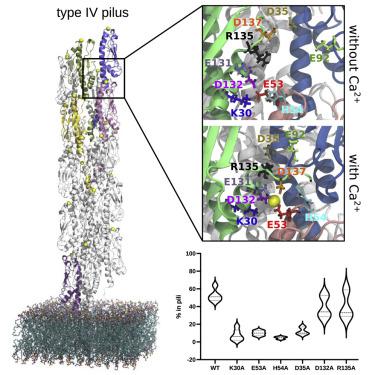
Type IV pili (T4P) are distinctive dynamic filaments at the surface of many bacteria that can rapidly extend and retract and withstand strong forces. T4P are important virulence factors in many human pathogens, including Enterohemorrhagic Escherichia coli (EHEC). The structure of the EHEC T4P has been determined by integrating nuclear magnetic resonance (NMR) and cryo-electron microscopy data. To better understand pilus assembly, stability, and function, we performed a total of 108 ms all-atom molecular dynamics simulations of wild-type and mutant T4P. Extensive characterization of the conformational landscape of T4P in different conditions of temperature, pH, and ionic strength is complemented with targeted mutagenesis and biochemical analyses. Our simulations and NMR experiments reveal a conserved set of residues defining a calcium-binding site at the interface between three pilin subunits. Calcium binding enhances T4P stability ex vivo and in vitro, supporting the role of this binding site as a potential pocket for drug design.
中文翻译:

IV型菌毛动力学和稳定性的计算和生化分析
IV 型菌毛 (T4P) 是许多细菌表面的独特动态细丝,可以快速伸展和收缩并承受强大的力量。T4P 是许多人类病原体的重要毒力因子,包括肠出血性大肠杆菌(EHEC)。EHEC T4P 的结构已通过整合核磁共振 (NMR) 和低温电子显微镜数据确定。为了更好地了解菌毛的组装、稳定性和功能,我们对野生型和突变型 T4P 进行了总共 108 毫秒的全原子分子动力学模拟。T4P 在不同温度、pH 和离子强度条件下的构象景观的广泛表征与靶向诱变和生化分析相辅相成。我们的模拟和核磁共振实验揭示了一组保守的残基,它们在三个菌毛蛋白亚基之间的界面处定义了一个钙结合位点。钙结合可增强 T4P离体和体外稳定性,支持该结合位点作为药物设计的潜在口袋的作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号