当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
E. coli Ribonucleotide Reductase β2 Subunit Inactivation by Triapine Occurs through Binding of a Triapine–Fe(II) Adduct
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2021-09-13 , DOI: 10.1021/acs.jpclett.1c02103 Mohammad S Safiarian 1 , R Atlee Watson 1 , Raquel L Lieberman 1 , Bridgette A Barry 1 , Adam R Offenbacher 2
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2021-09-13 , DOI: 10.1021/acs.jpclett.1c02103 Mohammad S Safiarian 1 , R Atlee Watson 1 , Raquel L Lieberman 1 , Bridgette A Barry 1 , Adam R Offenbacher 2
Affiliation
|
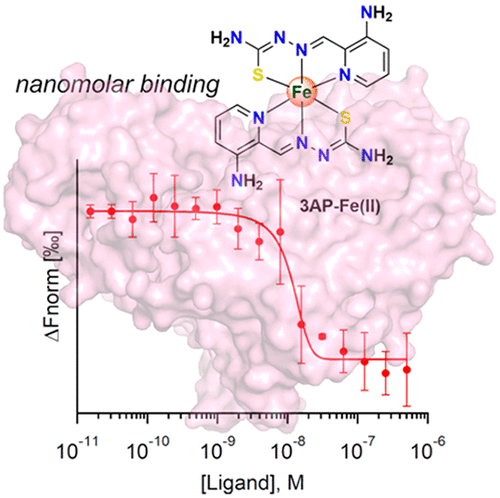
Ribonucleotide reductase (RNR), which supplies the building blocks for DNA biosynthesis and its repair, has been linked to human diseases and is emerging as a therapeutic target. Here, we present a mechanistic investigation of triapine (3AP), a clinically relevant small molecule that inhibits the tyrosyl radical within the RNR β2 subunit. Solvent kinetic isotope effects reveal that proton transfer is not rate-limiting for inhibition of Y122· of E. coli RNR β2 by the pertinent 3AP-Fe(II) adduct. Vibrational spectroscopy further demonstrates that unlike inhibition of the β2 tyrosyl radical by hydroxyurea, a carboxylate containing proton wire is not at play. Binding measurements reveal a low nanomolar affinity (Kd ∼ 6 nM) of 3AP-Fe(II) for β2. Taken together, these data should prompt further development of RNR inactivators based on the triapine scaffold for therapeutic applications.
中文翻译:

通过 Triapine-Fe(II) 加合物的结合发生大肠杆菌核糖核苷酸还原酶 β2 亚基失活
核糖核苷酸还原酶 (RNR) 为 DNA 生物合成及其修复提供基础材料,与人类疾病有关,并正在成为治疗靶点。在这里,我们介绍了三苯丙胺 (3AP) 的机理研究,这是一种临床相关的小分子,可抑制 RNR β 2亚基内的酪氨酰自由基。溶剂动力学同位素效应表明,质子转移不是相关 3AP-Fe(II) 加合物抑制大肠杆菌RNR β 2的 Y122· 的速率限制。振动光谱进一步证明,与羟基脲对 β 2酪氨酰基自由基的抑制不同,含有质子线的羧酸盐不起作用。结合测量显示低纳摩尔亲和力 ( Kd ∼ 6 nM) 的 3AP-Fe(II) 用于 β 2。综上所述,这些数据应该会促进基于三氮平支架的 RNR 灭活剂的进一步开发,用于治疗应用。
更新日期:2021-09-23
中文翻译:

通过 Triapine-Fe(II) 加合物的结合发生大肠杆菌核糖核苷酸还原酶 β2 亚基失活
核糖核苷酸还原酶 (RNR) 为 DNA 生物合成及其修复提供基础材料,与人类疾病有关,并正在成为治疗靶点。在这里,我们介绍了三苯丙胺 (3AP) 的机理研究,这是一种临床相关的小分子,可抑制 RNR β 2亚基内的酪氨酰自由基。溶剂动力学同位素效应表明,质子转移不是相关 3AP-Fe(II) 加合物抑制大肠杆菌RNR β 2的 Y122· 的速率限制。振动光谱进一步证明,与羟基脲对 β 2酪氨酰基自由基的抑制不同,含有质子线的羧酸盐不起作用。结合测量显示低纳摩尔亲和力 ( Kd ∼ 6 nM) 的 3AP-Fe(II) 用于 β 2。综上所述,这些数据应该会促进基于三氮平支架的 RNR 灭活剂的进一步开发,用于治疗应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号