当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Study of Decomposition Kinetics and Thermochemistry of Bis(dimethylamino)silane—Formation of Methyleneimine and Silanimine Species
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2021-09-13 , DOI: 10.1021/acs.jpca.1c04940 James M Stevenson 1 , Yujun Shi 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2021-09-13 , DOI: 10.1021/acs.jpca.1c04940 James M Stevenson 1 , Yujun Shi 1
Affiliation
|
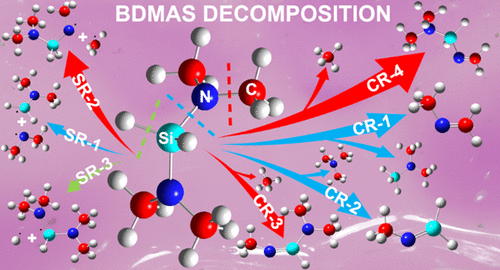
The gas-phase decomposition kinetics and thermochemistry of bis(dimethylamino)silane (BDMAS), a potential precursor in the chemical vapor deposition of silicon nitride and silicon carbonitride thin films, were systematically studied using ab initio calculations at the CCSD(T)/6-311 + G(2d,p)//B3LYP/6-311 + + G(d,p) level of theory. The reaction routes were mapped out, exploring both the concerted and stepwise reactions in three different zones with the initial cleavage of Si–N, N–Me (Me = CH3), and Si–H, respectively. It was found that the energy needed to break N–Me at 80.6 kcal·mol–1 is lower than the ones for Si–N and Si–H, both at 87.4 kcal·mol–1. When compared with tris(dimethylamino)silane (TrDMAS), it has been shown that the three bonds of N–Me, Si–N, and Si–H in BDMAS can be ruptured more easily, suggesting that BDMAS could be a more efficient precursor gas than TrDMAS. Upon decomposition, BDMAS tends to form methyleneimine and silanimine species, where four methyleneimine species and three silanimine species were produced. From the investigation of the effect of temperature on the kinetic and thermodynamic competition of different decomposition pathways, it has been demonstrated that the concerted formation of N-dimethylaminosilyl methyleneimine (H2C═N–SiH2NMe2) by the elimination of CH4 from BDMAS is the most kinetically and thermodynamically favored pathway in the whole temperature range from 0 K (ΔH0‡ = 62.7 kcal·mol–1 and ΔH0 = 7.7 kcal·mol–1) up to 2673 K. In addition, lower temperatures favor the production of N-methyl methyleneimime (H2C═NMe), whereas high temperatures promote the formation of N-methylsilanimine (H2Si = NMe) and 1-dimethylamino-N-methylsilanimine (Me2NSi = NMe).
中文翻译:

双(二甲氨基)硅烷分解动力学和热化学的理论研究——亚甲基亚胺和硅烷亚胺物种的形成
在 CCSD(T)/6 上使用 ab initio 计算系统地研究了双(二甲氨基)硅烷 (BDMAS)(氮化硅和碳氮化硅薄膜化学气相沉积的潜在前体)的气相分解动力学和热化学-311 + G(2d,p)//B3LYP/6-311 + + G(d,p) 理论水平。绘制了反应路线,探索了三个不同区域中的协同反应和逐步反应,分别是 Si-N、N-Me(Me = CH 3)和 Si-H的初始裂解。结果表明,在 80.6 kcal·mol –1 时分解 N-Me 所需的能量低于 Si-N 和 Si-H所需的能量,均为 87.4 kcal·mol –1. 与三(二甲氨基)硅烷 (TrDMAS) 相比,研究表明 BDMAS 中的 N-Me、Si-N 和 Si-H 三个键更容易断裂,表明 BDMAS 可能是更有效的前驱体气体比 TrDMAS。分解后,BDMAS 倾向于形成亚甲基亚胺和硅烷胺物种,其中产生了四种亚甲基亚胺物种和三种硅烷胺物种。通过研究温度对不同分解途径的动力学和热力学竞争的影响,已经证明通过消除 CH 4协同形成N-二甲氨基甲硅烷基亚甲基亚胺(H 2 C= N -SiH 2 NMe 2)在从 0 K(Δ H 0 ‡ = 62.7 kcal·mol –1和 Δ H 0 = 7.7 kcal·mol –1)到 2673 K的整个温度范围内,来自 BDMAS 的动力学和热力学最有利的途径。此外,较低的温度有利于生产的ñ甲基methyleneimime(H 2 C═NMe),而高温度促进的形成ñ -methylsilanimine(H 2的Si = NME)和1-二甲基氨基- ñ -methylsilanimine(ME 2 NSI = NME) .
更新日期:2021-09-23
中文翻译:

双(二甲氨基)硅烷分解动力学和热化学的理论研究——亚甲基亚胺和硅烷亚胺物种的形成
在 CCSD(T)/6 上使用 ab initio 计算系统地研究了双(二甲氨基)硅烷 (BDMAS)(氮化硅和碳氮化硅薄膜化学气相沉积的潜在前体)的气相分解动力学和热化学-311 + G(2d,p)//B3LYP/6-311 + + G(d,p) 理论水平。绘制了反应路线,探索了三个不同区域中的协同反应和逐步反应,分别是 Si-N、N-Me(Me = CH 3)和 Si-H的初始裂解。结果表明,在 80.6 kcal·mol –1 时分解 N-Me 所需的能量低于 Si-N 和 Si-H所需的能量,均为 87.4 kcal·mol –1. 与三(二甲氨基)硅烷 (TrDMAS) 相比,研究表明 BDMAS 中的 N-Me、Si-N 和 Si-H 三个键更容易断裂,表明 BDMAS 可能是更有效的前驱体气体比 TrDMAS。分解后,BDMAS 倾向于形成亚甲基亚胺和硅烷胺物种,其中产生了四种亚甲基亚胺物种和三种硅烷胺物种。通过研究温度对不同分解途径的动力学和热力学竞争的影响,已经证明通过消除 CH 4协同形成N-二甲氨基甲硅烷基亚甲基亚胺(H 2 C= N -SiH 2 NMe 2)在从 0 K(Δ H 0 ‡ = 62.7 kcal·mol –1和 Δ H 0 = 7.7 kcal·mol –1)到 2673 K的整个温度范围内,来自 BDMAS 的动力学和热力学最有利的途径。此外,较低的温度有利于生产的ñ甲基methyleneimime(H 2 C═NMe),而高温度促进的形成ñ -methylsilanimine(H 2的Si = NME)和1-二甲基氨基- ñ -methylsilanimine(ME 2 NSI = NME) .











































 京公网安备 11010802027423号
京公网安备 11010802027423号