当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ortho-Deuteration of Aromatic Aldehydes via a Transient Directing Group-Enabled Pd-Catalyzed Hydrogen Isotope Exchange
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-09-13 , DOI: 10.1021/acs.joc.1c01411 Junhua Kong 1, 2 , Zhi-Jiang Jiang 1 , Jiayuan Xu 3 , Yan Li 1, 2 , Hong Cao 1 , Yanan Ding 1 , Bencan Tang 3 , Jia Chen 1 , Zhanghua Gao 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-09-13 , DOI: 10.1021/acs.joc.1c01411 Junhua Kong 1, 2 , Zhi-Jiang Jiang 1 , Jiayuan Xu 3 , Yan Li 1, 2 , Hong Cao 1 , Yanan Ding 1 , Bencan Tang 3 , Jia Chen 1 , Zhanghua Gao 1
Affiliation
|
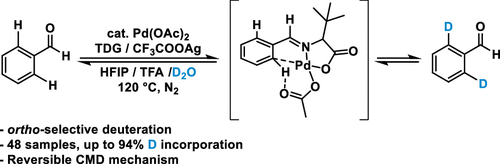
A practical and scalable ortho-selective deuteration of aromatic aldehydes was accomplished by Pd-catalyzed hydrogen isotope exchange with deuterium oxide as an inexpensive deuterium source. The use of tert-leucine as a transient directing group facilitates the exchange, affording a wide range of ortho-deuterated aromatic aldehydes with deuterium incorporation up to 97%. The control experiments suggest that the addition of silver trifluoroacetate resists the unexpected reduction of Pd(II), while the theoretical study indicates a rapid reversible concerted metalation–deprotonation process.
中文翻译:

通过瞬态导向基团启用的 Pd 催化氢同位素交换对芳香醛进行邻位氘化
通过与作为廉价氘源的氧化氘进行 Pd 催化的氢同位素交换,实现了芳香醛的实用且可扩展的邻位选择性氘化。使用叔亮氨酸作为瞬态导向基团促进了交换,提供了多种氘掺入率高达 97% 的邻位氘代芳香醛。对照实验表明,三氟乙酸银的加入可以抵抗 Pd(II) 的意外还原,而理论研究表明,这是一个快速可逆的协同金属化-去质子化过程。
更新日期:2021-10-01
中文翻译:

通过瞬态导向基团启用的 Pd 催化氢同位素交换对芳香醛进行邻位氘化
通过与作为廉价氘源的氧化氘进行 Pd 催化的氢同位素交换,实现了芳香醛的实用且可扩展的邻位选择性氘化。使用叔亮氨酸作为瞬态导向基团促进了交换,提供了多种氘掺入率高达 97% 的邻位氘代芳香醛。对照实验表明,三氟乙酸银的加入可以抵抗 Pd(II) 的意外还原,而理论研究表明,这是一个快速可逆的协同金属化-去质子化过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号