Analytica Chimica Acta ( IF 5.7 ) Pub Date : 2021-09-11 , DOI: 10.1016/j.aca.2021.339052 Fernanda C Pinheiro 1 , Miguel Ángel Aguirre 2 , Joaquim A Nóbrega 3 , Nerea González-Gallardo 4 , Diego J Ramón 4 , Antonio Canals 2
|
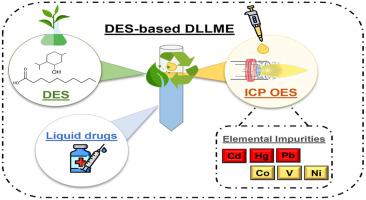
A simple, fast, sensitive and green pretreatment method for determination of Cd, Co, Hg, Ni, Pb and V in oral and parenteral drug samples using inductively coupled plasma optical emission spectrometry (ICP OES) has been developed. According to United States Pharmacopoeia (USP), those metals must be reported in all pharmaceutical products for quality control evaluation (i.e., elemental impurities from classes 1 and 2A of USP Chapter 232). To improve the analytical capabilities of ICP OES, a dispersive liquid-liquid microextraction (DLLME) has performed using a safe, cheap and biodegradable deep eutectic solvent (DES) as extractant solvent (a mixture of 2:1 M ratio of DL-menthol and decanoic acid). Seven parameters affecting the microextraction efficiency have carefully optimized by multivariate analysis. Under optimized conditions, the DES-based DLLME-ICP OES procedure improved limit of quantitation (LOQ) values on range from 12 to 85-fold and afforded an enrichment factor on average 60-times higher than those obtained to direct ICP OES analysis. Consequently, LOQ values for Cd, Co, Hg, Ni, Pb and V have been on average 10-times lower than target limits recommended for drugs from parenteral route of administration. Trueness has evaluated by addition and recovery experiments following USP recommendations for three oral drug samples in liquid dosage form and three parenteral drugs. Recovery and RSD values have been within the range of 90–109% and 1–6%, respectively. All analytes were below the respectives LOQ values, hence, lower than the limits proposed by USP Chapter 232.
中文翻译:

基于低共熔溶剂的分散液-液微萃取用于电感耦合等离子体发射光谱法测定口服和注射药物中的元素杂质
开发了一种使用电感耦合等离子体发射光谱法 (ICP OES) 测定口服和肠胃外药物样品中 Cd、Co、Hg、Ni、Pb 和 V 的简单、快速、灵敏和绿色的预处理方法。根据美国药典 (USP),必须在所有药品中报告这些金属以进行质量控制评估(即来自 USP 第 232 章第 1 和 2A 类的元素杂质)。为了提高 ICP OES 的分析能力,使用安全、廉价且可生物降解的低共熔溶剂 (DES) 作为萃取溶剂(DL-薄荷醇和 2:1 M 比例的混合物)进行了分散液-液微萃取 (DLLME)。癸酸)。通过多变量分析对影响微萃取效率的七个参数进行了仔细优化。在优化条件下,基于 DES 的 DLLME-ICP OES 程序将定量限 (LOQ) 值提高了 12 到 85 倍,并且提供的富集因子平均比直接 ICP OES 分析获得的高 60 倍。因此,Cd、Co、Hg、Ni、Pb 和 V 的 LOQ 值平均比肠胃外给药途径药物的推荐目标限值低 10 倍。按照 USP 建议,对三种口服液体剂型药物样品和三种肠胃外药物进行了添加和回收实验评估真实性。回收率和 RSD 值分别在 90-109% 和 1-6% 的范围内。所有分析物均低于各自的 LOQ 值,因此低于 USP 第 232 章提出的限值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号