Microporous and Mesoporous Materials ( IF 4.8 ) Pub Date : 2021-09-11 , DOI: 10.1016/j.micromeso.2021.111433 Zhicheng Ji 1 , Heyu Sun 1 , Yuanfeng Zhu 1 , Dongdong Zhang 1 , Lianhuan Wang 1 , Fengying Dai 1 , Yiping Zhao 1 , Li Chen 1
|
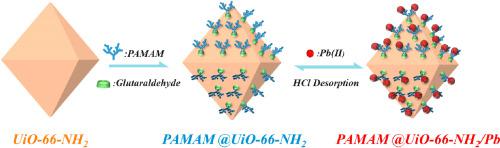
The pollution of water systems by lead ions poses a significant health hazard to humans and ecosystems. A new type of adsorbent was developed by grafting polyamidoamine (PAMAM) onto Zr(IV)-containing metal-organic frameworks (PAMAM@UiO-66-NH2), which possesses many adsorption sites and a high specific surface area. This novel adsorbent can efficiently reach the adsorption equilibrium of Pb(II) in 50 min, and the maximum adsorption capacity for lead ions was determined to be 334.32 mg/g. The effects of pH, adsorbent quality, contact time, temperature, and background ions on adsorption were assessed using batch adsorption methods. The experimental data agreed with pseudo-quadratic kinetics and the Freundlich model, which proved that the adsorption behaviour was controlled by multilayer chemical adsorption. Simultaneously, the adsorbent undergoes spontaneous endothermic reactions with Pb(II). PAMAM@UiO-66-NH2 demonstrated excellent selectivity in the removal of Pb(II) and maintained its removal efficiency even after being reused six times. Density functional theory calculations and characterisation analyses provided a quantitative basis for the selective removal of lead ions by PAMAM@UiO-66-NH2. It was also concluded that the adsorption strength of the N group toward Pb(II) was in the order of primary amino group > secondary amino group > tertiary amino group. All of these results clarified that PAMAM@UiO-66-NH2 could efficiently and selectively remove Pb(II) from aqueous solutions.
中文翻译:

使用功能化 PAMAM@UiO-66-NH2 纳米复合材料增强选择性去除铅离子:实验和机制
铅离子对水系统的污染对人类和生态系统构成了重大的健康危害。通过将聚酰胺胺(PAMAM)接枝到含Zr(IV)的金属有机骨架(PAMAM@UiO-66-NH 2),具有许多吸附位点和高比表面积。这种新型吸附剂可以在 50 分钟内有效地达到对 Pb(II) 的吸附平衡,对铅离子的最大吸附量为 334.32 mg/g。使用分批吸附方法评估 pH 值、吸附剂质量、接触时间、温度和背景离子对吸附的影响。实验数据符合拟二次动力学和Freundlich模型,证明吸附行为受多层化学吸附控制。同时,吸附剂与 Pb(II) 发生自发吸热反应。PAMAM@UiO-66-NH 2在去除 Pb(II) 方面表现出优异的选择性,并且即使在重复使用六次后仍保持其去除效率。密度泛函理论计算和表征分析为PAMAM@UiO-66-NH 2选择性去除铅离子提供了定量基础。还得出结论,N基团对Pb(II)的吸附强度为伯氨基>仲氨基>叔氨基。所有这些结果都说明PAMAM@UiO-66-NH 2可以有效且选择性地从水溶液中去除Pb(II)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号