iScience ( IF 5.8 ) Pub Date : 2021-09-11 , DOI: 10.1016/j.isci.2021.103117 Kaori Cho 1 , Takashi Ushiki 1, 2 , Hajime Ishiguro 1 , Suguru Tamura 1 , Masaya Araki 3 , Tatsuya Suwabe 1 , Takayuki Katagiri 1 , Mari Watanabe 2 , Yoko Fujimoto 2 , Riuko Ohashi 4, 5 , Yoichi Ajioka 4, 5 , Ippei Shimizu 6 , Shujiro Okuda 7 , Masayoshi Masuko 1 , Yoshimi Nakagawa 8 , Hideyo Hirai 9, 10 , Warren S Alexander 11, 12 , Hitoshi Shimano 3 , Hirohito Sone 1
|
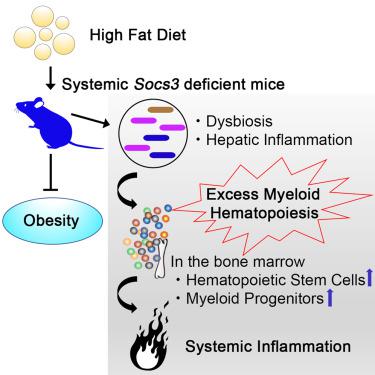
The suppressors of cytokine signaling (SOCS) proteins are negative regulators of cytokine signaling required to prevent excessive cellular responses. In particular, SOCS3 is involved in the regulation of metabolic syndromes, such as obesity and diabetes, by suppressing leptin and insulin signals. SOCS3 also suppresses the inflammatory response associated with metabolic stress, but this specific role remains undefined. Wild-type mice on a high-fat diet (HFD) exhibited only fatty liver, whereas systemic deletion of SOCS3 resulted in excessive myeloid hematopoiesis and hepatic inflammation. In addition, depletion of the gut microbiota resulted in considerable improvement in excess granulopoiesis and splenomegaly, halting the progression of systemic inflammation in SOCS3KO mice on the HFD. This result suggests that intestinal dysbiosis is involved in inflammation associated with SOCS3KO. Although contributing to diet-induced obesity and fatty liver, SOCS3 is nevertheless critical to suppress excess myeloid hematopoiesis and severe systemic inflammation associated with intestinal dysbiosis on HFD.
中文翻译:

高脂肪饮食改变的微生物群会加速与全身 SOCS3 缺乏相关的致死性骨髓造血
细胞因子信号转导 (SOCS) 蛋白的抑制因子是防止过度细胞反应所需的细胞因子信号转导的负调节因子。特别是,SOCS3 通过抑制瘦素和胰岛素信号参与代谢综合征的调节,如肥胖和糖尿病。SOCS3 还抑制与代谢应激相关的炎症反应,但这种特定作用仍未确定。高脂肪饮食 (HFD) 的野生型小鼠仅表现出脂肪肝,而 SOCS3 的系统性缺失导致骨髓造血过多和肝脏炎症。此外,肠道微生物群的消耗导致过量的粒细胞生成和脾肿大的显着改善,阻止了 HFD 上 SOCS3KO 小鼠全身炎症的进展。该结果表明肠道生态失调与 SOCS3KO 相关的炎症有关。尽管导致饮食诱导的肥胖和脂肪肝,但 SOCS3 对于抑制与 HFD 肠道生态失调相关的过量骨髓造血和严重全身炎症至关重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号