Journal of Molecular Graphics and Modelling ( IF 2.7 ) Pub Date : 2021-09-12 , DOI: 10.1016/j.jmgm.2021.108033 Isaac Ofori 1 , George Baffour Pipim 1 , Richard Tia 1 , Evans Adei 1
|
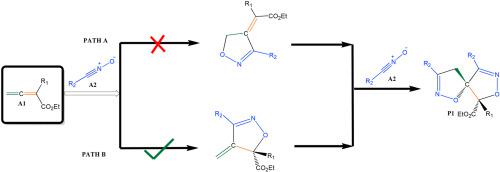
The molecular mechanism of the double (3 + 2) cycloaddition (32CA) reaction between nitrile oxides and allenoates has been studied using density functional theory at the M06-2X/6-311G (d,p) level of theory. In the first 32CA, the nitrile oxide adds chemo- and regio-selectively to the C–C double bond of the allenoate closest to the carboxylate group followed by a subsequent regioselective addition to the olefinic bond of the isoxazoline intermediate. The rate constant for the preferred pathway (formation of 4-methylene-2-isoxazoline intermediate) in the reaction of ethyl substituted allenoate and mesitonitrile oxide is 5.3 × 102 s−1 in THF which is about 13 times faster than the closest competing step (formation of its regioisomer 5-methylene-2-isoxazoline intermediate) which has a rate constant of 4.4 × 101 s−1. Strong electron-donating groups (EDGs) and electron-withdrawing groups (EWGs) decrease activation barriers and hence increase the reaction rate. Also, the dimerization of nitrile oxide to form furaxon is found to be kinetically unfavored.
中文翻译:

腈氧化物和烯丙酸酯双 (3 + 2) 环加成反应形成螺二异恶唑啉的 DFT 研究
使用密度泛函理论在 M06-2X/6-311G (d,p) 理论水平上研究了腈氧化物和烯丙酸酯之间双 (3 + 2) 环加成 (32CA) 反应的分子机制。在第一个 32CA 中,氧化腈将化学和区域选择性加成到最靠近羧酸酯基团的烯丙酸酯的 C-C 双键,然后随后区域选择性加成到异恶唑啉中间体的烯键上。取代的烯丙酸乙酯和氧化甲基腈反应中优选途径(4-亚甲基-2-异恶唑啉中间体的形成)的速率常数为5.3 × 10 2 s -1在 THF 中,这比速率常数为 4.4 × 10 1 s -1的最接近的竞争步骤(形成其区域异构体 5-亚甲基-2-异恶唑啉中间体)快约 13 倍。强给电子基团 (EDGs) 和吸电子基团 (EWGs) 降低了活化能垒,从而提高了反应速率。此外,发现氧化腈二聚形成呋喃酮在动力学上是不利的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号